We present a case of necrotizing fasciitis in a 66-year-old Caucasian woman with rheumatoid arthritis receiving tocilizumab, and provide a review of published cases. The patient exhibited no systemic symptoms and discreet cutaneous inflammatory signals at presentation. She was successfully treated with broad-spectrum empiric antibiotic therapy and surgical debridement.
Presentamos un caso de fascitis necrosante en una mujer caucásica de 66 años de edad con artritis reumatoide en tratamiento con tocilizumab y se ha realizado una revisión de casos publicados. La paciente ha presentado falta de síntomas sistèc)micos y señales inflamatorias cutáneas discretas. Ha sido tratada con èc)xito con terapia antibiótica empírica de amplio espectro y desbridamiento quirúrgico.
Necrotizing fasciitis (NF) is a rare severe infection affecting subcutaneous tissue and superficial fascia associated with considerable mortality (70•80%).1•3 The infections are the most feared adverse events of tocilizumab (TOC); there are few published pos-marketing data.
A case report of NF is presented with a published cases review through PubMed database and several European Biologics Registers.
Case reportA 66-year-old Caucasian woman with a seropositive RA presented with sudden onset of severe and progressive forearm and elbow pain with 3 days of evolution. She denied fever and history of trauma or animal bite. Since fourteen years ago, she has been consecutively treated with methotrexate (up to 20mg/week), etanercept (50mg weekly) and adalimumab (40mg fortnightly), which were discontinued owing to lack of efficacy. For three years, she has been treated with prednisolone (7.5mg/day), leflunomide (LEF) (10mg/day) and TOC (8mg/kg intravenous (iv) every 4 weeks). The prior medical history was significant for bacterial osteomyelitis (foot fifth digit) under TOC monotherapy for a year. Vital signs were as follow: pulse, 86beats/min; blood pressure, 135/72mmHg; body temperature, 36.8°C. Had active synovitis in metacarpophalangeal, interphalangeal and wrist joints. Left upper limb presented diffusely oedematous with a slight erythematous rash and increased temperature. Laboratory tests were as follow: hemoglobin 12.3g/dl; white blood cell (WBC) count 11.8í109/l (neutrophils 10.6í109/l); platelets 149í109/l; C-reactive protein (CRP) 74.6mg/L; creatinine 1.51mg/dl; sodium 138mEq/l and glucose 91mg/dl. Arteriovenous Doppler ultrasound excluded venous thrombosis.
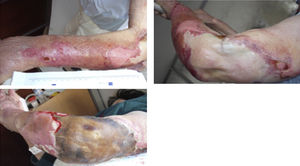
Three days later, erythrocyte sedimentation rate was 58mm and CRP 188.3mg/L. Differential diagnosis included cellulitis and septic arthritis. Immunosuppressants drugs were discontinued, cholestyramine and empiric antibiotic therapy (vancomycin and imipenem) were initiated. Magnetic resonance showed peri-aponeurosis and muscle edema (especially deltoid muscle), signs of cellulitis, without organized collections. At day 5 of treatment, lesions rapidly progressed to extensive areas of skin detachment and necrosis with severe exudation (Fig. 1). She had now periodic fever. Enterobacter aerogenes, Pseudomonas aeruginosa and Stenotrophomonas maltophilia were identified in the skin; blood cultures were negative. Surgical debridement with subsequent partial skin graft and a twenty-days period of antibiotics led to progressive improvement. She was discharged at day 39.
After one-year follow-up there was no recurrence of infections. She is under hydroxychloroquine (400mg/day) and prednisolone (7.5mg/day). Methotrexate was added but soon discontinued due to oral mucositis. TOC was permanently withdrawn.
Literature review and discussionWe identified three case reports of NF in RA patients receiving TOC3•5 and three under TNF antagonists6•8 (Table 1). Published rates of serious skin and soft tissue infections in RA patients vary widely between different European Biologics Registers, from 12•18 per 1000 patient-years in the British register to 37•76 per 1000 patient-years in German register.9 Results from British register showed that the majority were cellulitis (227/309; 73%) and only four cases were NF (all with anti-TNF).10
Characterization of reported cases of necrotizing fasciitis with tocilizumab and TNFα blockers.
| Case (reference) | 15 | 23 | 36 | 47 | 58 | 69 |
| Biological | TCZ | TCZ | TCZ | Etanercept | Infliximab | Etanercept |
| Gender | F | F | M | M | M | F |
| Age (years) | 59 | 65 | 53 | 39 | 54 | 37 |
| RA duration (years) | 3 | 15 | ? | 2 | 12 | 21 |
| TNFα blockers/TCZ therapy duration (months) | 2 | 12 | 48 | 24 | ? | 7 |
| Current cDMARD | No | No | ? | AZA | i.m MTX | No |
| Previous DMARD | MTX, HCQ, adalimumab, etanercept | MTX, SSZ, tracolimus, infliximab, etanercept | ? | No | SSZ, AZA, cyclosporin, i.m. gold, MTX | Gold, HCQ, SSZ, MTX, AZA, LEFL |
| CCTs | No | ? | ? | No | ? | Yes |
| DM | No | No | No | No | No | No |
| Previous infection under biologics | No | No | No | No | No | No |
| Area | Pectoral region Shoulder | Hand, Forearm | Leg | Shoulder | Thigh | Lower limb |
| Fever at apresentation | No | No | No | Yes | No | No |
| WBC (/mm3) | Normal | 5300 | 22,100 | Normal | 14,820 | Normal |
| Neutrophils (/mm3) | ? | 4900 | 19,669 | Raised | 13,990 | ? |
| CRP (mg/dl) | Normal | 0.04 | 25.3 | Raised | ? | ? |
| Fasciitis in CT/MRI | Yes/not done | Not done/not done | Yes/not done | Yes/not done | Not done/not done | Not done/not done |
| Isolated agents in skin swabs/blood cultures | Group A Streptococcus (pyogenes)/None | Group A Streptococcus/none | Group A Streptococcus/none | No/No | Group A Streptococcus/Group A Streptococcus | Group A Streptococcus/Group A Streptococcus |
| Treatment | Gentamicin, penicillin, clindamycin, (+IVIGs+hydrocortisone) | ? | Penicillin G, meropenem | Cefotaxime, metronidazol, vancomycin, ciprofloxacin; clindamycin, ciprofloxacin, flucloxacillin, benzylpenicillin | DIC treatment | Clindamicina, vancomicina, levofloxacina, IVIGs |
| Surgical debridement/skin graft | Yes/no | Yes/no | Yes/yes | Yes/no | Yes/yes | Yes/no |
| Sepsis/death | Yes/yes | Yes/no | No/no | No/no | Yes (DIC)/yes | Yes/yes |
AZA, azathioprine; cDMARD, classic disease-modifying antirheumatic drugs; CCT, corticotherapy; CRP, C-reactive protein; CT, computerized tomography; DIC, disseminated intravascular coagulation; DM, diabetes mellitus; F, female; HCQ, hydroxychloroquine; IVIG, intravenous immunoglobulin; LEFL, leflunomide; MRI, magnetic resonance imaging; M, male; MTX, methotrexate; RA, rheumatoid arthritis; SSZ, sulfasalazine; TCZ, tocilizumab; WBC, white blood cell; ?, unknown.
A German cohort study concluded that longer disease duration, history of severe infections, previous exposure to multiple DMARDs and concomitant treatments with LEF, prednisone or proton-pump inhibitor increase the risk of infections under TOC. This is our patient profile. In the TOWARD study, infections were more prevalent among patients receiving TOC and DMARDs simultaneously; however, there was no explicitly assigned association to LEF.11 Thus, we cannot overlooked that our patient was under LEF and glucocorticoids and we should attribute the adverse event to their association with TOC rather than to TOC alone. TOC contribution remains speculative and causality can neither be asserted nor ruled out with certainty. So, we believe that is important to report such infections in the post-marketing surveillance.
In conclusion, physician should keep in mind that infections may mimic a disease flare and the absence of fever does not exclude serious infections in RA patients under biologics.
Ethical disclosuresProtection of human and animals subjectsThe authors state that no human or animal experiments have been performed for this investigation.
Confidentiality of dataThe authors state that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that they have received written consent from the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflict of interestAll authors declare no conflicts of interest.