Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by the presence of autoantibodies. Research on the pathogenic mechanisms involved in systemic autoimmune diseases has largely focused on the involvement of the adaptive immune system with dysregulated responses of T and B cells. However, in recent years, there is increasing evidence of the significant role played by the innate immune system, particularly neutrophils, in these diseases, particularly in RA. Neutrophil extracellular traps (NETs) are extracellular structures composed of remodeled and concentrated chromatin with DNA, histones, and neutrophil proteins, and were first described in 2004. It has been studied that NETs may play a pathogenic role in RA and could be a source of autoantigens, increasing the immune response in the form of autoantibodies in this disease. The possible role of NETs and other markers of neutrophil activation as biomarkers of activity in RA and other immune-mediated diseases has also been studied.
This article reviews the role of NETs in RA. It discusses the role of neutrophils and the latest advances in NETs, especially their involvement in autoimmune phenomena in RA. Finally, a literature review is conducted on the determination of NETs in peripheral blood and their relationship as a biomarker of RA activity, as well as their potential role in disease monitoring.
La artritis reumatoide (AR) es una enfermedad autoinmune sistémica caracterizada por la presencia de autoanticuerpos. Las investigaciones sobre los mecanismos patogénicos implicados en las enfermedades autoinmunes sistémicas se centran en gran medida en la participación del sistema inmune adaptativo con las respuestas desreguladas de las células T y B. Sin embargo, en los últimos años, aumenta la evidencia del importante papel que juega del sistema inmune innato, en particular los neutrófilos, en estas enfermedades, particularmente en la AR. Las trampas extracelulares de neutrófilos (NETs) son estructuras extracelulares compuestas por cromatina remodelada y concentrada con ADN, histonas y proteínas de los neutrófilos y son un mecanismo de acción de los neutrófilos que se describió por primera vez en 2004. Se ha estudiado que pueden desempeñar un papel patogénico en la AR y podrían ser fuente de autoantígenos e incrementar la respuesta inmunológica en forma de autoanticuerpos en esta enfermedad. También se ha estudiado el posible papel de las NETS y otros marcadores de activación neutrofílica como biomarcadores de actividad en la AR y otras enfermedades inmunomediadas.
En el presente artículo se revisa el papel el papel de las NETs en la AR. Se revisa el papel del neutrófilo y los últimos avances en NETs, especialmente en su participación en los fenómenos de autoinmunidad en la AR. Finalmente se hace una revisión de la literatura sobre la determinación de NETs en sangre periférica y su relación como biomarcador de actividad de la AR y su posible papel para monitorización de la enfermedad.
Autoimmune rheumatic diseases make up a group of inflammatory disorders that occur when the immune system loses its ability to distinguish self from non-self, which results in an inflammatory response that damages various tissues and organs such as the kidney, blood vessels, and joints.1 These diseases include rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), anti-neutrophil cytoplasmic antibodies associated vasculitis (AAV), antiphospholipid syndrome (APS), and idiopathic inflammatory myopathies (IIM). Systemic autoimmune diseases are very heterogeneous in their clinical presentation, but have in common that they are characterised by significant dysregulation of innate and adaptive immune responses and that many of them are associated with the development of autoantibodies targeting intra- and extracellular antigens.2
Research on the pathogenic mechanisms involved in systemic autoimmune diseases largely focuses on dysregulated T- and B-cell responses. However, it is inappropriately activated neutrophils that have the greatest potential to cause local tissue damage, both by their presence in large numbers in foci of inflammation and by their releasing their cytotoxic content directly into host tissues.3 In recent years, there has been mounting evidence of the importance of these cells in the inflammatory response in systemic autoimmune diseases.
The predominant role of neutrophils in autoimmunity is evidenced by significant neutrophil infiltration of affected tissues and the generation of autoantibodies targeting their components. Neutrophil dysfunction has been observed, manifested by elevated production of reactive oxygen species (ROS) and cytokines, such as tumour necrosis factor α (TNF-α) and interleukin-6 (IL-6),4,5 as well as inappropriate activation of the complement cascade and dendritic cells.6 In autoimmune diseases, this dysfunction generates a highly inflammatory environment leading to antigenic modifications of proteins, which in turn triggers the activation of adaptive immune cells and the production of autoantigen-specific antibodies.7 In RA in particular, there is growing evidence that neutrophils play a very important role in both the initiation and perpetuation of the disease, through their direct effects on the synovial membrane and their role in modulating the immune response.8,9
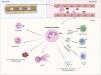
Neutrophils: physiological roles in the immune responseNeutrophils are the most abundant leukocytes in the circulation. They are specialised cells of the innate immune system that play a key role in host defence against micro-organisms. Recent technological advances have shown that neutrophils are much more versatile and heterogeneous than previously thought. It is now recognised that they are not only effector cells against invasive pathogens, but also play an active role in orchestrating the progression of inflammation by regulating the functions of other cells of the innate and adaptive immune system.10
Neutrophils originate in the bone marrow from resident myeloid precursors in response to granulocyte colony-stimulating factor. Cell adhesion molecules, such as integrins and selectins, are considered essential in the process of neutrophil egress from the bone marrow. Under homeostatic conditions and in the absence of inflammatory stimuli, mature neutrophils patrol the bloodstream, where they remain for a relatively short period (6−12h) before dying. During this time in circulation, they contribute to various physiological functions such as angiogenesis, coagulation, and tissue repair.11 If the circulating neutrophil encounters a danger signal (microbes, pathogen metabolites, tissue damage products) it is activated and responds primarily to molecules classified as pathogen-associated molecular patterns or danger-associated molecular patterns through a variety of pattern recognition receptors, including Toll-like receptors (TLRs). Interleukin-8 (IL-8) is the cytokine that acts as the main chemokine that signals neutrophil migration into an inflammatory environment.11
Neutrophils "shoot at first sight" and rapidly eradicate invasive pathogens by a variety of strategies. These include phagocytosis, degranulation (through the release of granular antimicrobial peptides, such as myeloperoxidase [MPO], neutrophil elastase [NE], and matrix metalloproteinases), or ROS production through the activation of NADPH oxidase.12 They also play an important role as regulators of the adaptive immune response by secreting a wide range of cytokines and chemokines that can regulate the function of almost all other immune cells. Activated neutrophils increase the expression of plasma membrane receptors, such as major histocompatibility complex class II, allowing them to present antigens to T cells. In addition, the uptake of apoptotic neutrophils by dendritic cells increases levels of antigen presentation.7 As regards the B cells, neutrophils synthesise cytokines crucial for their development, such as B-cell activating factor or proliferation-inducing ligand A (Fig. 1).7
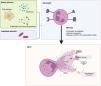
Neutrophil extracellular traps (NETs)In 2004, a new mechanism of action of neutrophils was described: neutrophil extracellular traps (NETs).13 NETs are defined as extracellular structures containing DNA, histones, and neutrophil proteins such as MPO, NE, calprotectin, or calgranulin, which are expelled from neutrophils in response to infectious or inflammatory stimuli. During NET formation, neutrophils undergo chromatin remodelling through histone processing by proteases and the remodelled chromatin then binds to antimicrobial proteins and enzymes and is released into the extracellular milieu to form a network (Fig. 2). NETs are produced in inflammatory areas and have a short half-life due to degradation by local DNases.10
The events leading to NET production rather than phagocytosis are not clear, nor is there consensus on the mechanisms by which they are generated.14 At least 2 methods of chromatin decondensation leading to NET formation (NETosis) have been described: NOX2-dependent and NOX2-independent.15 NOX2-dependent NETosis, also called suicidal NETosis, occurs through the activation of NOX2 and intraphagosomal ROS generation. This causes an increase in intracellular membrane permeability, NADPH oxidase stimulates MPO, which induces the displacement of NE to the nucleus, where histones are processed and degraded, leading to chromatin decondensation and NET release.16 Suicidal NETosis is thus a type of cell death distinct from apoptosis and necrosis. Necrosis occurs when cells suffer extreme damage, and an unscheduled cell death occurs. Apoptosis, on the other hand, is a process of programmed cell death that plays an important role in the elimination of damaged or potentially dangerous cells. In contrast, NETosis is a defence mechanism that is activated in response to the presence of pathogens and other inflammatory stimuli (Table 1).17,18
Differential characteristics between the 3 types of cell death: NETosis, necrosis, and apoptosis.
| NETosis | Necrosis | Apoptosis | |
|---|---|---|---|
| Process | Programmed cell death that occurs in response to infection or inflammation | Unprogrammed cell death due to extreme damage to cells | Programmed cell death that occurs in individual cells in response to internal or external signals |
| Condition | Physiological | Pathological | Physiological/pathological disturbances |
| Cell change | It breaks down and releases its contents, forming a network of fibres | Oedema and cell lysis with release of cell contents | Retraction and shrinkage. It fragments, releasing apoptotic bodies, which are phagocytosed by other cells |
| Cell nucleus | Decondensation of nuclear chromatin with disintegration of nucleus and union with cytoplasmic components | Disintegration of the membrane | The nucleus condenses and DNA is fragmented |
| Inflammatory reaction | Yes | Yes | No |
NOX2-independent NETosis does not require the production of ROS by NOX2. In this case, mitochondrial ROS combine with the increased intracellular calcium level to activate peptidyl arginase deiminase (PAD) enzymes (e.g., PAD4), leading to histone hypercitrullination, chromatin decondensation, and NET release10 (Fig. 3).
Several inflammatory agents have been reported to induce NET release, such as IL-8, lipopolysaccharide, nitric oxide, or TNF-α.10 It is believed that, depending on the stimulus, the type of NETs produced will differ, as will their functions.19
During the resolution phase of inflammation, NETs are eliminated once they have fulfilled their defensive function. This clearance is mediated by phagocytic cells and other mechanisms, which degrade and remove the structures from the extracellular milieu. Signals that promote NET clearance include the production of anti-inflammatory factors, such as IL-10 and resolvin D1, and activation of the low-density lipoprotein receptor-related protein 1 pathway in phagocytic cells, which promotes phagocytosis and degradation of NETs. DNases are enzymes that normally degrade DNA released by apoptotic cells to prevent inflammation. They also play an important role in the elimination of NETs. In particular, DNase1, present in the lysosomes of phagocytic cells, which is able to degrade the DNA present in NETs. DNase deficiency has been implicated in the accumulation of NETs in tissues and the perpetuation of inflammation in autoimmune diseases and other chronic inflammatory disorders.20
The persistence of NETs and lack of clearance contribute to the chronicity of inflammation and perpetuation of disease.19 Adequate regulation of NET clearance is therefore essential to maintain immune system homeostasis and prevent chronicity of inflammation.
NETs and autoimmunityFor years, systemic autoimmune diseases were mainly associated with defects in adaptive immune responses. However, in the last 2 decades, several studies have highlighted the important role of cells of the innate immune system, such as neutrophils, in these diseases.21,22 In particular, special attention has been paid to the formation of NETs as a neutrophil activation state associated with autoimmunity.23
NETs are an alternative defence mechanism used by neutrophils to capture and possibly eliminate microbes, but they also play an important role in regulating immune response and maintaining the body's homeostasis.13 Excessive production of NETs and inappropriate activation of immune cells can have negative consequences and contribute to the pathogenesis of different immune-mediated inflammatory diseases.
NETs carry immunostimulatory proteins that activate other immune cells and promote the induction of signalling pathways important for immune response.24 They contain many molecules released by neutrophils, including autoantigens, which are known targets of systemic autoimmunity. These autoantigens include post-translationally modified proteins, such as histones, which have been found to be methylated, acetylated, carbamylated, and citrullinated3,25; double-stranded DNA, or proteins such as MPO, proteinase 3 (PR3), or NE.10
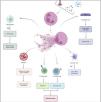
The peptides present in NETs stimulate activation of the NLRP3 inflammasome in macrophages, leading to the release of inflammatory cytokines such as IL-1 and IL-18, which in turn promote neutrophil activation and the formation of more NETs. In addition, they are able to activate plasmacytoid dendritic cells causing them to synthesise type I interferons (IFN-I) and trigger the release of pro-inflammatory cytokines such as IL-6 and TNF-α.26,27 On the other hand, NETs have been reported to promote the polarisation of T cells towards a pro-inflammatory phenotype, which contributes to the induction of the systemic inflammatory response.20 NET-bound proteins, such as matrix metalloproteinases and histones, promote vasculopathy by damaging endothelial cells. NETs, in turn, promote tissue factor expression, which activates platelets and clotting factors, thereby promoting thrombosis. Autoantibodies, immune complexes, autoantigens, complement activating factors, and cytokines can activate neutrophils and induce NETosis (Fig. 4).
Relationship between NETs and autoimmunity. NETs carry immunostimulatory proteins that can activate other immune cells (macrophages, dendritic cells, lymphocytes); they also contain autoantigens that can trigger autoantibody-mediated autoimmune responses. Furthermore, NET-bound proteins can damage endothelial cells and promote thrombosis. Autoantibodies, immune complexes, autoantigens, complement activating factors, and cytokines can activate neutrophils and induce NETosis.
Figure created with Biorender.
Exacerbated NETosis has been linked to several systemic autoimmune diseases, such as RA, SLE, AAV, IIM, and APS, and to thrombus formation in patients with rheumatic diseases.28 A combination of factors affecting neutrophil activation, NET formation and clearance, and the production of modified autoantigens contribute to the development of these diseases.
An imbalance between NET formation and clearance has been observed in SLE patients, which plays an important role in the perpetuation of autoimmunity, disease exacerbation, and organ manifestations.29 Many proteins present in NETs also correlate with SLE disease activity and play a direct role in tissue damage.3 NETs have been detected in the skin and glomeruli of SLE patients.10 Several studies have found alterations in the activity of DNases in these patients.30,31 Decreased NET degradation is considered to mark an early step in the pathogenesis of the disease and to be a key factor in the breakdown of normal immune tolerance.32
Exacerbated NETosis has also been observed in AAV, with increased NETs found in the circulation of these patients.33 Patients with AAV produce autoantibodies against neutrophil cytoplasm-localised proteins (ANCA), mainly targeting PR3 and MPO,34 molecules present in NETs. In addition, it has been reported that ANCA-stimulated neutrophils can induce NETosis.35
In patients with IIM, higher levels of circulating NETs have been found due to increased NETosis and incomplete degradation, probably mediated by the presence of serum antibodies against DNase1.36 It has been suggested that the detection of NETs in blood could be useful in assessing myopathy activity, as an association with specific disease manifestations and severity has been found.37,38 In addition, experimental in vitro studies have found that myositis-specific antibodies, including antibodies against MDA5, can directly induce NET formation.39
APS is another immune-mediated disease associated with NETosis. Neutrophils in patients with APS have an inflammatory phenotype that favours vascular thrombus formation, which enhances neutrophil binding to endothelial cells in the vascular wall.40 NET formation in blood vessels forms a scaffold for platelet aggregation and thrombus accumulation, further damaging endothelial cells and contributing to the up-regulation of adhesion molecules.28 Patients with APS have an elevated level of cell-free DNA and NET debris in the circulation and in affected tissues, due to the ability of serum and antibodies of APS patients to promote NET formation and inhibit the degradation of these structures.41
Overall, these observations suggest that dysregulation of neutrophil pathways and NET formation may have a pathogenic role in immune-mediated diseases, including the induction of autoimmune responses and tissue damage during disease activity, and that detecting NET-derived products in peripheral blood may be useful. However, the current evidence is limited, and more studies are needed.
Neutrophils in rheumatoid arthritisRA is a chronic, inflammatory, systemic autoimmune disease characterised by inflammation, synovial hyperplasia and destruction of articular cartilage.42 Because of its systemic autoimmune nature, RA has systemic manifestations, such as vascular or pulmonary disorders.43 Two families of autoantibodies characteristic of RA have been described: rheumatoid factor, an antibody targeting the Fc portion of immunoglobulin, and anti-citrullinated protein/peptide antibodies (ACPA).7
Neutrophils contribute to inflammation and tissue damage when inappropriately activated by cytokines, chemokines, and autoantibodies.44 In recent years, there has been mounting evidence to describe neutrophils as critical cells in the pathogenesis of RA and there has been a resurgence of interest in the role of these cells in the last decade.14 Experimental models of RA have clearly demonstrated that neutrophil depletion or functional inhibition is capable of decreasing inflammation and bone damage.19
It has been found that neutrophils of RA patients have a significantly different functionality to those from healthy individuals. These cells are in a pro-inflammatory state predisposed to ROS production, unlike circulating neutrophils in healthy individuals. In addition, notable differences in gene and protein expression have been identified between peripheral blood neutrophils of RA patients and their healthy counterparts, including higher levels of TNF and PR3.9
Activated neutrophils are found in large numbers in both RA synovial fluid and pannus and have the potential to cause joint damage. They are the predominant cells in the synovial fluid of RA patients and secrete a repertoire of cytokines and chemokines, including RANK ligand (RANKL) and BAFF (also known as BLyS), which are involved in the activation of osteoclasts and B lymphocytes, respectively.14 In addition, neutrophils in RA synovial fluid express MHC class II molecules and present antigen to T lymphocytes, an immune function they share with macrophages and dendritic cells.
Conditions in the synovial joint, such as hypoxia and the presence of anti-apoptotic cytokines (such as TNF, macrophage colony-stimulating factor, and granulocyte colony-stimulating factor, or IL-8) as well as autoantibodies, increase neutrophil survival for up to several days.9,45 This sustained activation causes host tissue damage through ROS release and degranulation of cytotoxic proteins. In RA, neutrophil granule proteins are found in high concentrations in synovial fluid and are responsible for cartilage and tissue damage, activation of soluble cytokines and receptors, inhibition of chondrocyte proliferation, and activation of synoviocyte proliferation and invasion. Importantly, these proteins act synergistically to perpetuate inflammation and joint damage in RA.9
Activated neutrophils also secrete S100A9/S100A8 (calprotectin), a protein member of the S100 protein family that plays an important role in the development of the inflammatory cascade in RA as trigger chemotaxis, phagocyte migration, and modulation of various macrophage functions. This acts as an important pro-inflammatory factor of innate immunity, such as molecular patterns associated with endogenous hazards through TLR4 activation, by which pathway they can also induce NETosis.46 The role of calprotectin as a biomarker of RA activity and as a prognostic marker of radiological progression and therapeutic response to targeted specific antirheumatic therapy (tsDMARDs) is well known and studied.47–52
NET in rheumatoid arthritisIn recent years there has been increasing interest in the role of NETs and NETosis in RA. It is thought that these structures may have an inflammatory role in the disease, both locally in the joint and in other tissues,53 and may be involved in the initiation and perpetuation of the disease through exposure to autoantigens.53–55 Neutrophils obtained from RA patients show increased spontaneous NET formation and this correlates with ACPA levels.53,56
One of the main predisposing factors to exacerbated NETosis is the pro-inflammatory milieu present in RA-affected tissues. This inflammatory environment generated by different cells, such as synovial fibroblasts (FLS), dendritic cells, and macrophages, which release pro-inflammatory cytokines such as TNF-α, IL-1β, and interferon-γ (IFN-γ), activates neutrophils and promotes their migration into inflamed joints. Once there, neutrophils are subjected to continuous stimulation by the pro-inflammatory environment, triggering NET release. Exposure of neutrophils to RA patient sera, especially samples with elevated levels of ACPA and rheumatoid factor, as well as the inflammatory cytokines IL-17A and TNF, have been shown to induce NET formation in isolated neutrophils of RA patients. In addition, neutrophils of RA patients have an increased susceptibility to NET formation compared to neutrophils from healthy individuals when stimulated.53
ACPAs are highly specific to RA and bind to different proteins, such as modified vimentin, histones, fibrinogen, and α-enolase. These antibodies are of various subtypes and form pathogenic immune complexes in the joints, where they promote inflammation and bone erosion.57 Neutrophils are an important source of citrullinated antigens, as they produce enzymes, such as PAD4, that catalyse the modification of arginine to citrulline. Experimental studies have shown that histone citrullination via PAD4 is an essential step in chromatin decondensation during NET formation.58
PAD enzymes are present in NETs in an active form and are detectable in rheumatoid synovium. In the presence of calcium, they modify proteins in the extracellular space in addition to their intracellular targets, thus creating additional epitopes for ACPA generation.24 In the synovial membrane, NET products, including citrullinated proteins, can be internalised by FLS, which increase expression of MHC class II molecules and present NET-derived peptides to CD4+T cells, creating a link to the adaptive immune response in RA.59 Indeed, intra-articular administration of NET-loaded LFS to humanised mice leads to the induction of antigen-specific T-cell responses, ACPA generation, and joint damage,60 supporting the idea that the interaction between FLS and NET in vivo has pathogenic consequences. In addition, NET-derived NE alters cartilage structure and promotes its citrullination, which may lead to synovial inflammation by increasing its immunogenicity and the production of autoantibodies.61
NETs have also been described as an important source of carbamylated proteins in RA patients.62 Anti-carbamylated protein antibodies (anti-CarP) are common in RA patients and are associated with increased joint destruction, mortality, and interstitial lung disease.63,64 These antibodies form immune complexes that activate bone resorption by osteoclasts, indicating a causal relationship with bone damage in RA.25 Recently, in a study conducted in HLA-DRB1*04:01 mice, it was shown that administration of NETs with carbamylated proteins stimulated rapid differentiation of monocytes to osteoclasts, mediated by TLR4 signalling and NET-associated proteins, including histones and NE. In addition, carbamylated histones were found to increase the number of osteoclasts and correlated with active bone resorption and inflammatory markers in plasma and synovial fluid. These results also indicate that NETs play a direct role in RA-associated bone erosion.65
In short, neutrophils appear to play a central inflammatory role in RA, both in the joint and probably in other tissues as well. It is possible that they play a role in initiating and perpetuating antigen exposure through the formation of NETs, as these structures are an important source of modified self-antigens that can promote proinflammatory responses as well as pathogenic adaptive immunity.
Detecting NETs in rheumatoid arthritisHow to detect NETsThe ability to detect NETs could be a potential complementary biomarker for patients with diseases with a higher rate of NET formation, such as RA. There is no standardised way of testing for NETs. They can be detected in fluids such as blood or sputum.66
The ability to detect NETs could be a potential complementary biomarker for patients with diseases with a higher rate of NET formation, such as RA. There is no standardised way of testing for NETs. They can be detected in fluids such as blood or sputum.66
It is difficult to identify and quantify NETs in peripheral blood because of their short lifetime due to degradation by local DNases. The most commonly used technique to determine NETs in plasma is their indirect detection, with NET-derived products, such as circulating free DNA, or NET-derived DNA-product complexes such as citrullinated histones (as citH3), NE, and MPO.66,67 The most commonly used technique for detecting NETs in peripheral blood is the enzyme-linked immunosorbent sandwich assay (ELISA).67 This method involves using specific antibodies to detect the levels of NET-associated proteins. In addition, ELISA assays are practical for detecting NETs in large cohorts of patients and clinical samples, as they allow several patients to be studied at the same time.13,26,68,69
Available evidence suggests that quantitative detection of NET-derived products could be a useful complementary tool for identifying individuals with RA and for monitoring patients.70 However, for NETs to serve as a screening tool, studies are needed to standardise and define normal and pathological levels.
Diagnostic value of identifying NETs in peripheral bloodSome recent studies have focused on detecting possible signalling pathways leading to increased NET formation in RA. This is to determine whether the products of NET formation are diagnostically useful.54 Levels of NET degradation products, such as circulating free DNA, free nucleosomes, NE-DNA complexes, and MPO-DNA complexes, are increased in the peripheral blood of RA patients.71
In vitro studies show that in RA there is a spontaneous increase in NET formation.72 Detecting NET-derived products in peripheral blood, such as free nucleosomes or DNA-MPO complexes, shows high value for the diagnosis of RA, with high sensitivity and specificity.56,72 The serum level of anti-NET antibodies (ANETA) is significantly higher in RA, especially in rheumatoid factor positive patients.73
Quantitative detection of NET-derived products could be a useful complementary tool to identify individuals at risk and to monitor RA patients. Several experimental studies have found that peripheral blood levels of NETs are higher in RA than in healthy controls,56,71,74–76 and therefore it is considered that they may have some complementary diagnostic value in suspected cases of RA.71
NETs as markers of disease activityThe search for serum biomarkers of inflammatory activity in RA patients is an area of special interest and the subject of much research in recent years. The classical acute phase reactants (CRP and ESR) do not always reflect the degree of active synovitis in RA patients and are influenced by a variety of factors.77 Neutrophil activity markers may be useful as biomarkers that more accurately reflect the disease status of RA patients. Calprotectin (S100A8/S100A9) is a protein with inflammatory activity that is mainly expressed in neutrophils and released into the extracellular milieu in response to inflammatory stimuli.78 It is present in large amounts in rheumatoid synovium.79 It can be determined in plasma and serum, and correlates closely with inflammatory activity in RA. Calprotectin appears to more accurately reflect the clinical status of the patient, including clinical remission or low disease activity.51,52,80 It is also a prognostic biomarker of radiological progression,80 a marker of therapeutic response to certain targeted DMARDs81 and is useful for predicting disease flare.82 Calprotectin would more accurately reflect the inflammatory status (measured as degree of synovitis) of RA patients treated with tocilizumab (TCZ) or JAK inhibitors than CRP.49,50
Measurement of NETs as a marker of neutrophil activity with utility as a potential marker of RA activity is an emerging trend in research. A review of the literature on the relationship between NETs and RA activity has been conducted (Appendix B Appendix A). Table 2 presents the different studies analysing the usefulness of measuring NET and NETosis in peripheral blood as a marker of disease activity in RA patients.
Summary of articles included in the review.
| Design | Study method NET/NETosis in peripheral blood | Results (NET and RA activity) | Notes | |
|---|---|---|---|---|
| Oliveira et al.74 | Retrospective cross-sectional study with 164 patients with RA and 76 healthy controls | MPO-DNA complex ELISA (Quant-iT™ PicoGreen® dsDNA Assay Kit [Invitrogen, Carlsbad, U.S.A.]). NET concentration in plasma | Patients with high disease activity have higher plasma NET concentrations compared to individuals with low disease activity. No statistically significant difference between moderate and high or moderate and low. | Study assessing periodontal disease. NET concentrations are also directly associated with clinical periodontal parameters |
| Luque-Tévar et al.75 | Prospective study with 104 patients with RA and 29 healthy controls.The RA patients were patients with conventional DMARD failure, without previous anti-TNF use and who were going to start treatment with anti-TNF.Assessment to place before and at 3 and 6 months after initiation of treatment | ELISAPLUS (Roche, Sigma-Aldrich, St Louis, USA) in serum using monoclonal antibodies directed against double-stranded DNA or histone.IF ELISA NE-DNA complex (HumanPMN Elastase ELISA Kit (Abcam, Cambridge, UK) in serum | Both NET remnants correlated with clinical, inflammatory, and oxidative stress parameters in RA patients. Patients who respond to anti-TNF treatment show a greater reduction in the parameters under study (including NETosis). Positive correlation between changes in molecular parameters and improvement in activity as measured by DAS28 | |
| Wang et al.84 | Prospective study with 74 patients with RA and 50 healthy controls | ELISA MPO-DNA complexes in serum (PicoGreen dsDNA Quantitation Kit (American Invitrogen) | No correlation between reduction in NET levels and DAS28 | Study evaluating acupuncture as an adjuvant therapy to treat RA (associated with conventional DMARDs) |
| Bach et al.76 | Multicentre study with 2 parts: cross-sectional: 2 RA cohorts and health control group; longitudinal: 205 RA with mean follow-up of 8 years. Healthy control group | MPO-DNA ELISA (measure absorbance with BioTEK). In plasma | No association between plasma NETs and RA activity. In seropositives, it did have a sensitivity of 68.6 and specificity of 75% to differentiate remission of disease activity. CRP did not, leading to the conclusion that it may have a role in monitoring disease activity, especially in seropositive patients | |
| Wang et al.56 | Cross-sectional study of 74 RA patients and 50 healthy controls | ELISA MPO-DNA complexes in serum | No association with clinical and laboratory activity parameters. | |
| Kaneko et al.62 | Retrospective case-control study with 40 patients with RA and periodontitis; 30 patients with periodontitis and 43 healthy controls | ELISA MPO-DNA complex (Cell Biolabs Laboratories, INC. San Diego, USA). Serum | Significantly positive correlation between NETs and DAS28-CRP | Study assessing periodontal disease. NET concentrations are also directly associated with periodontal clinical parameters. Study showing for the first time that circulating levels of CarP and NET are associated with the severity of periodontitis and are influenced by periodontal treatment in RA patients |
| Pérez-Sánchez et al.71 | Study in two parts: cross-sectional with 106 patients and 40 healthy controls; prospective: 2 cohorts with RA treated with tocilizumab or with infliximab, followed for 6 months | ELISA quantification of free nucleosomes in serum using ELISA PLUS (Roche Diagnostics, Switzerland) using monoclonal antibodies to double-stranded DNA or histones | Patients not on biologics: NETs are higher if disease is active. ROC curves show that they serve to identify active patients.Direct relationship between cell-free nucleosome levels and clinical, inflammatory (CRP and ESR), activity (DAS28) and oxidative stress levels. Anti-TNFa and anti-IL-6R decreased NETosis and reduced its inflammatory profile. | |
| Ruiz-Limón et al.85 | Prospective study.Twenty patients with active RA and failure of conventional DMARDs, who were going to start biologic treatment with tocilizumab | ELISA quantification of free nucleosomes in serum using ELISA PLUS (Roche Diagnostics, Basel, Switzerland) using monoclonal antibodies to double-stranded DNA or histones | Decreased release of cell-free nucleosomes in the serum of RA patients after treatment with tocilizumab | First study to assess the direct effect of tocilizumab on monocytes and neutrophils in RA patients |
| Fagerhol et al.83 | Cross-sectional study; 100 patients with multiple myeloma; 8 patients with RA | IF ELISA dual hybrid in plasma:anti-calprotectin/S100A12 and anti-calprotectin/histone complexes | Positive correlation between CRP and NET in RA patients |
CarP, anti-carbamylated protein antibodies; CRP, C-reactive protein; DAS28, disease activity score; DMARD, disease modifying drug; ESR, erythrocyte sedimentation rate; MPO-DNA, myeloperoxidase-DNA complexes; NE-DNA, neutrophil elastase-DNA complexes; NETs, neutrophil extracelular traps; RA, rheumatoid arthritis.
Several studies have been conducted to assess whether NETs can be used as a marker of disease activity in RA patients and have found conflicting results. Some studies have found a relationship between levels of NET remnants in peripheral blood and disease activity,62,71,74,75 while in other studies this association has not been confirmed.56,83,84
Pérez-Sánchez et al. analysed NETs in peripheral blood (ELISA nucleosomes and NE and MPO) in 106 RA patients and observed that NET-derived complexes were strongly correlated with clinical disease activity (measured by DAS28 and with acute phase reactants, both CRP and ESR), as well as correlating with markers of oxidative stress. Using ROC curve analysis, the concentration of NETs was used to identify patients with active RA.71 Another study, also using the detection of nucleosomes by ELISA and monoclonal antibodies against nucleosomes, in addition to NE-DNA complexes, showed a positive correlation with activity parameters. Two other studies using MPO-DNA complex detection to study NETs in patients with RA and periodontal disease found an association between NET levels and the composite RA activity indices DAS28-ESR74 and DAS28-CPR, although it was only moderate.62
However, other groups report negative results. In 2016, Fagerhol et al. published an innovative study evaluating the use of a new hybrid ELISA method to detect anti-calprotectin/S100A12 complexes and anti-calprotectin/histone (NET-derived products) in patients with 2 inflammatory diseases: RA and multiple myeloma.83 This study included only 8 patients with RA. They found a moderate positive correlation between NET remnant levels and CRP only. However, they found no association with any other activity parameter, either clinical or laboratory.83 A cross-sectional study of 74 RA patients and 50 healthy controls evaluating the relationship between serum NET remnant levels (measuring MPO-DNA complex by ELISA) and different activity indices in RA patients found no correlation with disease activity.56 A study in a Chinese population also found no association between NET levels and RA activity.84
NETs and therapeutic responseIn 2016, Ruiz-Limón et al. designed a study to assess the effect of TCZ on prothrombotic factors in RA. NETs were among the parameters studied. They included 20 patients with active RA who had never received biologic DMARDs, who were prescribed TCZ for 6 months. Serum nucleosome concentration was determined using an ELISA to detect nucleosomes in serum. They found improvement in RA activity after initiation of TCZ treatment and that this was associated with a reduction in NETs. This result was consistent with that observed in in vitro studies. Exposing isolated neutrophils from patients to TCZ prior to the initiation of biologic therapy confirmed the specificity of the effects of this therapy on the reduction of neutrophil NETosis. This was the first study to evaluate the direct effect of TCZ on neutrophils and NETs of patients with RA.85
A later work used NETs to evaluate the effects of biologic drugs such as anti-TNF (infliximab) and anti-IL-6 receptor (TCZ) on NET formation.71 They studied 106 patients with RA. They found that both biologic drugs had a significant effect on NETosis inhibition (with a lower concentration of NET derivatives in peripheral blood) with a parallel reduction in inflammatory activity as well as in the expression of key inflammatory mediators after 6 months of treatment. These results coincided with in vitro experiments, finding that exposure of neutrophils from healthy controls and RA patients to RA patient plasma promoted a significant increase in DNA fibre extrusion and that this could be prevented by exposure to infliximab and TCZ.71
A study published in 2020 proposed an integrative model of disease assessment according to changes in serological parameters related to inflammation, NETosis, oxidative stress, and micro-RNAs after anti-TNF treatment. Patients responding to anti-TNF treatment had a reduction in inflammatory parameters, including NET concentration (determining NE-DNA complexes and nucleosomes).75
NET-derived products, therefore, may have a role in the assessment of therapeutic efficacy in RA patients.
NETs and periodontitisThe association between RA and periodontal disease has been the focus of numerous investigations due to their common pathological features.86 NETs are an antimicrobial mechanism that could play a role in the pathogenesis of gingivitis and periodontitis.87 There is some evidence of a connection between NETs, periodontal disease, and RA.88
In 2018 Kaneko et al. showed that the concentration of MPO-DNA complex (NETs-derived product) in peripheral blood was associated with the severity of periodontitis and RA. They found a positive, albeit moderate, correlation between NET-derived products and RA activity measured by DAS28-CRP. Treatment of periodontitis led to a reduction in the levels of NETs and RA activity measured by DAS28-CRP. These differences were statistically significant, although clinically insignificant. Notably, this is the first study to link anti-carbamylated antibody levels with NETs and periodontal disease in patients with RA.61
In a recent cross-sectional study72 in 164 RA patients, plasma NET concentrations were found to correlate with clinical parameters of periodontal disease and RA activity. Non-surgical periodontal treatment was shown to be effective in reducing plasma NETs, coinciding with improved clinical parameters of these diseases.
LimitationsThere are several limitations to comparing studies on NETs as a marker of RA activity.
Firstly, the variety of measurement methods used in the different studies. Although there are recommendations, we do not have a standardised method for measuring NETs, and therefore there is no homogeneity in the research papers, which makes comparison difficult. However, the study of NETosis in peripheral blood with the quantification of NET remnants using different protein complexes is complex and is only an indirect assessment of the process. Furthermore, the protein complexes used to measure NET remnants may not be specific for NETosis products, which can lead to false positives or negatives.
It should also be considered that neutrophils are highly active cells sensitive to external stimuli and that the manipulation of peripheral blood during sample collection and processing may trigger their activation and NET generation. Spontaneous NET generation during sample collection and processing will result in overestimation of NET levels in peripheral blood and thus misinterpretation of results. Therefore, it is important to take measures to minimise spontaneous NET generation during sample collection and processing.
Another limitation is the small sample size of some studies, which limits the generalisability of the results to the population. Several are exploratory studies. In addition, the patient populations included in the studies were different in type of RA, disease duration and severity, previous treatment, among others, which may affect the results. There is a lack of studies in specific populations, with particular attention, in our opinion, to the treatments received by the patients. In view of the results observed, we believe that biologic drugs play a relevant role in NETosis.
Furthermore, longitudinal studies are lacking. Most studies are cross-sectional and therefore do not allow us to follow the long-term evolution of the disease or to establish a cause-effect relationship between NET levels and disease activity.
ConclusionsSome studies in recent years suggest that innate immunity, especially neutrophils, may play an important role in the pathogenesis of RA and other immune-mediated diseases. Recent research shows that the NETosis process and the formation of NETs could be a pathway involved not only in the defence against pathogens but also responsible for the exposure to autoantigenic proteins with a relevant role in the development of autoimmunity, as well as in the perpetuation of the inflammatory process. However, more studies are needed to confirm that NETs play a major role in the pathogenesis of the disease and are not just a secondary finding associated with the inflammatory process. The role of NET detection in monitoring disease activity and the effect of different anti-rheumatic therapies is yet to be determined. The role of neutrophils, and more specifically the NETosis process, will continue to be an area of growing interest in rheumatic disease research in years to come.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Mercedes Guerra Rodríguez, documentalist of the Spanish Society of Rheumatology, for her help with the literature search.