Although antineutrophil cytoplasmic antibody (ANCA) vasculitis has a variety of clinical manifestations, valvular compromise is not common, especially in anti-myeloperoxidase (anti-MPO) antibody (perinuclear [P]-ANCA) vasculitis. We report the case of a 38-year-old woman with ANCA-associated vasculitis who was diagnosed with valve vegetation, that resolved with immunosuppressive therapy.
Aunque las vasculitis asociadas a ANCA tienen manifestaciones clínicas diversas, la afección valvular es infrecuente, especialmente en el contexto de una poliangitis microscópica. Reportamos el caso de una mujer de 38 años con vasculitis ANCA con características clínicas de poliangitis microscópica y anticuerpos anti-mieloperoxidasa positivos (MPO), que presentó una masa vegetante en la válvula mitral que resolvió con inmunosupresión.
Valve involvement in antineutrophil cytoplasmic antibody (ANCA) vasculitis is rare. However, there are around 20 cases reported in the literature, most in the context of granulomatosis with polyangiitis and c-ANCA. Infective endocarditis and ANCA vasculitis can have similar clinical manifestations that include: purpura, glomerulonephritis, pulmonary and ocular involvement, elevated acute phase reactants and fever; these impede diagnosis and appropriate treatment. In addition, ANCA can be present as an epiphenomenon in endocarditis which can add to diagnostic confusion. We describe the case of a patient with non-infective endocarditis associated with ANCA which, because of its clinical features and antibodies, behaved like a microscopic polyangiitis, and resolved with immunosuppression without requiring valve replacement.
CaseA previously healthy 38-year-old woman consulting with anasarca, on admission high blood pressure and gradual creatinine elevation (increase from 1mg/dl to 6mg/dl in one month) were recorded. The anti-nuclear antibodies were negative, complement was normal and no infections were revealed. Positivity for ANCA was found by indirect immunofluorescence (IIF) with P pattern 1:160, and the ANCA by ELISA anti-myeloperoxidase were also positive. Kidney biopsy showed pauci-immune, focal segmental necrotising glomerulonephritis with extracapillary proliferation (30% fibrocellular crescents). No other organs were affected, CT of the thorax and transthoracic ultrasound were normal. Initially a diagnosis of ANCA vasculitis limited to the kidney was considered. Glucocorticoids were started: first, pulse methylprednisolone (500mg intravenously/every day/3 days), followed by oral prednisolone 60mg/day; in addition, plasma exchanges (she received 14 in total), and cyclophosphamide 500mg intravenously on day 0 and day 15. After one month's hospitalisation the patient was discharged without requiring haemodialysis, with average creatinine levels of 2mg/dl. The patient was prescribed an outpatient dose of prednisolone 50mg/day, and a third dose of cyclophosphamide.
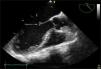
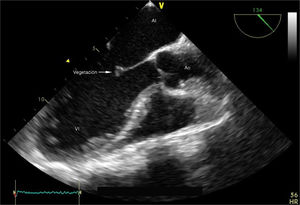
However, she was readmitted after 8 days with dyspnoea, cough and fever, and assured us that she had not stopped taking the glucocorticoids. Echocardiogram showed a vegetating mass of 4×5mm in the A2 scallop of the anterior valve of the mitral valve with failure, which showed no independent movement (Fig. 1). Because of the suspicion of infective endocarditis, the patient was started on empirical broad-spectrum antibiotics (vancomycin and piperacillin-tazobactam, adjusted to her renal function), but her blood cultures were negative. On admission, CT of the thorax was normal. Despite the antibiotic treatment the patient deteriorated clinically, with increased dyspnoea until she went into ventilatory failure and required admission to the intensive care unit. A further CT of the thorax showed findings suggesting alveolar haemorrhage, which was confirmed with bronchoalveolar lavage, and a repeat echocardiograph showed the persistence of the vegetative valve lesion and mitral failure. At that time repeat ANCA by ELISA were performed which were positive (anti-MPO).
Considering the patient's clinical deterioration despite management as infective endocarditis and the development of a new clinical manifestation attributable to vasculitis (alveolar haemorrhage) the possibility of valve involvement by ANCA vasculitis was considered, and that the combined clinical symptoms corresponded to a microscopic polyangiitis (MPA). After finding similar cases of valve involvement in ANCA vasculitis, the patient was given the third dose of cyclophosphamide (500mg) and started on rituximab. The patient recovered, her blood cultures remained negative and on discharge echocardiography showed resolution of the vegetative lesion and the valve failure.
DiscussionANCA vasculitis has heterogeneous clinical manifestations, most of which are serious. Valve involvement is possible, as evidenced by this clinical case, and poses a diagnostic challenge, especially when establishing the difference with infective endocarditis. Manifestations by septic embolism and subacute bacterial endocarditis (SBE) can also simulate endocarditis associated with ANCA.1 The literature reports 10%–33% positive ANCA in patients with infective endocarditis (generally c-ANCA by IIF and anti-proteinase 3 [anti-PR3] by ELISA).1–3
It might help to establish the difference between infective endocarditis and ANCA vasculitis to consider that the majority of patients with SBE and ANCA as an epiphenomenon were positive for other antibodies and had evidence of circulating immune complexes such as hypocomplementaemia or vascular complement deposits, which are absent in ANCA vasculitis. It has also been described that ANCA in endocarditis become negative after antibiotic treatment and, contrary to the cases of ANCA vasculitis with valve lesions, the need for valve replacement is rare.1
In the case we describe the diagnosis of MPA was based on the presence of lung/kidney syndrome, positivity to anti-MPO antibodies by ELISA and p-ANCA by IFI with no ENT involvement or demonstrated granulomatous disease.
In the cases of endocarditis due to ANCA vasculitis reported in the literature, valve involvement is most commonly aortic regurgitation, followed by mitral regurgitation (as in the case we describe), both secondary to: valve perforation, valve or septum thickening or the presence of masses or vegetations. We should highlight that vegetations have not been described in all cases.4,5
Table 1 describes the main features of other cases reported in the literature on endocardial involvement in patients over the age of 16 due to vasculitis associated with ANCA.
Features of Reported Cases of Endocarditis Due to ANCA Vasculitis.
| Year of publication | Age | Sex | ANCA (ELISA) | ANCA (IIF) | Type of vasculitis | Type of valve involvement | |
|---|---|---|---|---|---|---|---|
| Levine | 1957 | 28 | M | ND | – | GPA | Mitral regurgitation |
| Dabbagh | 1982 | 16 | M | ND | – | GPA | Aortic regurgitation |
| Gerbracht | 1987 | 20 | F | ND | ND | GPA | Aortic regurgitation |
| Yanda | 1989 | 77 | F | ND | ND | GPA | Aortic and mitral regurgitation |
| Fox | 1994 | 20 | M | ND | ANCA+ND of | GPA | Aortic and mitral regurgitation |
| Davenport 1 | 1994 | 19 | M | PR3 | c-ANCA | GPA | Aortic regurgitation |
| Davenport 2 | 1994 | 53 | M | PR3 | c-ANCA | GPA | Aortic regurgitation |
| Grant | 1994 | 32 | M | ND | c-ANCA | GPA | Aortic and mitral regurgitation |
| Goodfield | 1995 | 25 | M | ND | c-ANCA | GPA | Aortic regurgitation |
| Anthony | 1999 | 48 | M | PR3 | c-ANCA | GPA | Aortic stenosis. Vegetation |
| Paik | 1999 | 48 | M | ND | c-ANCA | GPA | Aortic vegetation |
| Leff | 1999 | 17 | M | ND | c-ANCA | GPA | Aortic regurgitation |
| Bruno | 2000 | 63 | F | ND | ANCA+ND of | GPA | Aortic regurgitation |
| Mishell | 2002 | 65 | M | PR3 | c-ANCA | GPA | Aortic and mitral vegetation |
| Stöllberger | 2003 | 56 | M | PR3 | – | Unknown | Aortic regurgitation. Vegetation |
| Herbst | 2003 | 56 | F | ND | – | GPA | Mitral regurgitation. Mitral mass |
| Ramakrishnan | 2004 | 44 | F | ND | c-ANCA | GPA | Mass mitral prosthesis, atrial side |
| Attaran | 2010 | 52 | M | ND | ND | GPA | Mitral regurgitation and stenosis. Mitral mass |
| Koyalakonda | 2010 | 52 | M | ND | – | GPA | Aortic and mitral regurgitation - mitral stenosis. Mitral mass |
| Lacoste | 2011 | 44 | M | MPO | p-ANCA | GPA | Aortic regurgitation |
| Muñoz-Grajales | 2017 | 38 | F | MPO | p-ANCA | MPA | Mitral regurgitation. Vegetation |
ANCA: antineutrophil cytoplasmic antibodies; IIF: indirect immunofluorescence; GPA: granulomatosis with poliangiitis; MPO: anti- myeloperoxidase; ND: no data; c-ANCA: cytoplasmic fluorescence pattern; p-ANCA: perinuclear fluorescence pattern; MPA: microscopic polyangiitis; PR3: proteinase 3; −: negative.
In half of the reported cases of valvular disease due to ANCA vasculitis, valve involvement presented concomitantly with the diagnosis of vasculitis, but in almost 30% it presented when the patient was already receiving immunosuppression. An important difference with SBE with positivity for ANCA, mentioned earlier, is that in most cases due to vasculitis valve replacement has been required and only in 2 cases, such as ours, immunosuppression was enough to resolve the valve involvement.4–6
We stress that as far as we know the reported cases of ANCA vasculitis with valve involvement presented in patients with a diagnosis of granulomatosis with polyangiitis, formerly Wegener's granulomatosis, and ours would be the first case described with microscopic polyangiitis.
ConclusionsAlthough rare, there are several cases in the literature that, like our case, highlight the possibility that vasculitis associated with ANCA might affect the heart valve apparatus. Establishing the difference between acute bacterial endocarditis and vasculitis associated with ANCA is a diagnostic challenge. Blood cultures, response to antibiotics, positivity to other antibodies, evidence of circulating immune complexes, and the persistence of ANCA can help the clinician to establish the difference, an infective process favouring the former and an ANCA vasculitis the latter.
Ethical DisclosuresProtection of people and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of InterestsThe authors have no conflict of interest to declare.
Please cite this article as: Muñoz-Grajales C, Chavarriaga JC, Márquez JD, Pinto LF. Endocarditis no infecciosa en poliangitis microscópica: reporte de un caso con respuesta exitosa a inmunosupresión. Reumatol Clin. 2019;15:e21–e23.