The association between microbes and joints has existed since antiquity, and remains complex. Diagnosis is often difficult to determine despite highly suspicious clinical characteristics for the presence of an underlying infection. Over the several past decades, considerable advances have occurred in diagnostic methodologies and therapy. However, the morbidity and mortality of septic arthritis remain high. Great advances have occurred in the diagnosis, pathogenesis, and therapeutic management of reactive arthritis, and there is evidence that when the responsible microorganism is Chlamydia trachomatis, complete remission and cure are possible. Emergent infections, especially viral, have been recognized, that is, HIV, hepatitis C, and most recently Chikungunya virus, and in the case of HIV associated articular manifestations, the introduction of HAART has resulted in a decrease in the incidence and development of newer complications such as the immune reconstitution syndrome. The infectious etiology of rheumatoid arthritis is being strongly considered once again, and the exciting association with periodontal disease is at the forefront of intense research. The gut microbiota is also being investigated and new and most interesting data are being gathered of the potential role of commensal gut organisms and the pathogenesis of rheumatoid arthritis.
La asociación entre las infecciones y la inflamación articular ha existido desde tiempos remotos y continúa siendo compleja. El diagnóstico es frecuentemente difícil de determinar, a pesar de la presencia de hallazgos clínicos que sugieren un proceso infeccioso. En las últimas décadas, han ocurrido considerables avances en metodología tanto diagnóstica como terapéutica. Sin embargo, la morbilidad y la mortalidad de la artritis séptica siguen siendo altas. Grandes adelantos han ocurrido en el diagnóstico, patogénesis y manejo terapéutico de la artritis reactiva, y hay evidencia de que cuando el patógeno responsable es Chlamydia trachomatis puede haber remisión completa y la cura es posible. Algunas infecciones emergentes, especialmente virales han sido reconocidas, por ejemplo, virus de inmunodeficiencia humana (VIH), de la hepatitis C, y más recientemente, el virus de Chickengunya. En los casos de VIH, la asociación de manifestaciones articulares y la introducción de la terapia antrirretroviral altamente activa (TARAA) han resultado en una disminución en la incidencia y el desarrollo de nuevas complicaciones, tales como el síndrome de reconstitución inmunológica. La infección como causa de la artritis reumatoide (AR) está siendo considerada nuevamente y la asociación con enfermedad periodontal ha vuelto a ser tema de gran interés e investigación. La microbiota intestinal también está siendo investigada y nuevos e interesantes datos han surgido, como es el papel potencial de los organismos comensales en el intestino como agentes causales en la AR.
The association between infections and joint inflammation has existed since ancient times, and by the period of Hippocrates the presence of joint inflammation in young men after their first sexual experience had already been described. This implied the existence of septic or reactive arthritis, possibly secondary to gonococcal infection, either intestinal or from the urinary tract in origin. The relationship between infection and arthritis was critically analyzed in a symposium organized by the World Health Organization with the Arthritis and Rheumatism Research Council in London in the year 1974.1 The agreed consensus classified the relationship between infection and joint involvement in four major categories. Group 1, infectious arthritis or septic arthritis, was defined by the presence of infection somewhere in the body and identification of the infectious agent or microbial product in the joint, Group 2, post infectious arthritis, characterized by the presence of infection and identification of bacterial antigens in the joint, for example arthritis secondary to meningococcemia; Group 3, reactive arthritis, defined as a joint inflammatory process in which the infection is known, originating either in the urinary or digestive tract, but where bacterial product is not detected in the joint; and Group 4, characterized by a joint syndrome or inflammatory process in which neither the infectious agent nor their products or antigens are detected or identified in the joint, for example, rheumatoid arthritis. Ziff, in the same symposium, mentioned four infections clearly associated with rheumatic disease: rheumatic fever, polyarthritis secondary to hepatitis B, Reiter's syndrome and Whipple's2 disease.
The advances that have occurred in recent decades, especially with the identification of infectious agents and their antigens through molecular biology, genetic and immunological tests, make it imperative to update the relationship between infection and arthritis. In this review we will try to update our knowledge on the relationship between infection and arthritis, emphasizing the current status of four topics of interest: septic or infectious arthritis, reactive arthritis, arthritis associated with infection by the human immunodeficiency virus (HIV) and infection in the pathogenesis of rheumatoid arthritis (RA).
Septic or Infectious ArthritisSeptic arthritis remains one of the few emergencies in rheumatology, and despite the great advances occurred in microbiology, molecular biology, imaging and therapy, morbidity and mortality have not changed dramatically over the past 25 years.
The epidemiology of septic arthritis is difficult to study for several reasons, especially because of the difficulty in establishing a precise bacteriological diagnosis in a good number of cases and the fact that most of the studies are retrospective and case3 series. The annual incidence has been reported, especially in European countries, to be between 4 and 10 per 100000 patients per year, and up to 30–100 cases per 100000 population in patients with chronic diseases, such as RA, joint replacement cases, elderly patients, immunocompromised populations and indigenous communities.4–8 The incidence is increasing in recent years due to a number of factors, including antimicrobial resistance, an aging population, increased use of invasive procedures and inmunosuppressed9 patients.
Risk factors (Table 1) have been largely unchanged over time. Extremes of life, the elderly and children, preexisting joint disease, patients with compromised immune status, patients on hemodialysis or who use intravenous drugs, diabetes, skin infections, orthopedic procedures such as arthroscopy or intra-articular injections are important predisposing factors for septic arthritis.9–11
Septic Arthritis: Risk Factors.
| Immune suppression or compromise |
| Children, elderly patients |
| Diabetes mellitus |
| History of joint disease: rheumatoid arthritis |
| Prosthetic joint |
| Hemodialysis |
| Use of intravenous drugs |
| Skin ulcers/Skin infection |
| Blood disease-sickle cell anemia |
| Use of intravenous catheters |
Septic arthritis most commonly occurs as a result of hematogenous dissemination into the synovium after an episode of bacteremia, facilitated by its high vascularity and because the synovial membrane has a basement membrane. Rarely, however, joint infection can occur as a consequence of an intraarticular injection or during joint aspiration.
Tarkowski et al.’s studies in animal models have provided a better understanding of bacterial and host factors in the pathogenesis of septic arthritis.12 In the experimental model of septic arthritis mediated by Staphylococcus aureus (S. aureus), the hematogenous inoculation of the bacterium induced, after 24h, septic arthritis in almost 100% of mice, with joint changes very similar to those occurring in humans.12 In this model, the authors found that gene depletion of macrophage-derived cytokines, such as lymphotoxin-α, tumor necrosis factor (TNF) receptor and interleukin-1 (IL-1), decreases the host protection against infection by S. aureus, which causes an increase in morbidity and mortality.13 Similarly, increased susceptibility to infectious arthritis due to a reduction in pathogen elimination articulación14 has been demonstrated in IL-10 deficient murine models. Moreover, mice deficient in IL-4 has been shown to decrease the incidence and mortality, which has been attributed to the role of IL-4 on bacterial growth, promoting the reduction in bacteria removal from the joint space.15 The activity of this cytokine in the pathogenesis of septic arthritis in humans has not been well studied.
The extracellular virulence factors have been studied in murine experimental models performed by Tarkowski13 and some of them have an important role in the development of erosive joint damage in septic arthritis. Components of the bacterial cell wall also modulate bacterial virulence. For example, studies of S. aureus protein A-deficient strains were associated with less severe disease in mice.16 Some strains of S. aureus, which are positive for the cytotoxin virulence Panton-Valentine leukocidin (PVL), which allows them to survive in neutrophils, are associated with fulminant infections, including joint infections in previously healthy patients with a higher rate of complications than PVL17 negative strains. Increased PVL positive MRSA strains were associated with an increased frequency of septic arthritis in the USA.18
S. aureus and Streptococcus microbial agents are more commonly associated with the development of septic arthritis, including children. There is great concern in the U.S. and some European countries due to the increase in the prevalence of S. aureus resistant to methicillin (MRSA), particularly that associated with patients who use intravenous drugs, the elderly and patients with infections related to orthopedic procedures.19 Gram-negative infections are increased in populations over.20 Gonococcal joint infection occurs less frequently in certain Western countries compared with African and Australian Aboriginal communities.8,21
The clinical characteristics of septic arthritis have not changed greatly; they includes patients with HIV infection.22 The presence of an especially large, swollen, red and hot joint, is reason enough to include septic arthritis in the differential diagnosis. The vast majority of patients (>75%) present monoarticular involvement, but in recent years polyarticular involvement is seen more frequently, especially in patients with risk factors. In children, septic arthritis of the hip is quite difficult to differentiate from transient synovitis. For several years, the Kocher criteria were used to establish the diferential23 diagnosis. When the 4 criteria are present, that is, difficulty in tolerating body weight, fever over 38.5°C (101.3°F), erythrocyte sedimentation rate greater than 40mm/h and leukocyte counts in peripheral blood in excess of 12000cells/mm3, the probability of septic arthritis reaches 99.6%. However, more recent work which incorporates a fifth criterion, C-reactive protein (CRP)≥20mg/l, showed that when the 5 criteria were present, predictive probability was only 59.9%, and fever was the best predictive factor of septic24 arthritis.
The gold standard for the diagnosis of septic arthritis remains the isolation of bacteria in synovial fluid and, though less useful, the detection in other tissues, especially in the blood. These methods, however, lack sensitivity and cultures take time to establish the diagnosis. In recent years, faster and more sensitive techniques have been introduced, such as those based on DNA by mass spectroscopy or polimerase25 chain reaction. These molecular techniques, however, are not sufficiently sensitive and reproducible to be used in clinical practice.
Among laboratory tests routinely used, CRP appears to be more useful for diagnosis than erythrocyte26 sedimentation rate.
The clinical dilemma frequently observed in patients with preexisting rheumatic diseases, such as RA, is the differential diagnosis with septic arthritis. Very recently, a Japanese research group has reported that expression of the neutrophil marker CD64 is quite useful in differentiating septic arthritis in patients with RA. They showed an increase of this marker in infected joints, with a sensitivity of 76% and specificity of 94.4%.27
A high index of suspicion and early initiation of antibiotic therapy, as well as drainage of the joint, are necessary for a good therapeutic response in septic arthritis. The choice of antibiotics should be guided by susceptibility patterns. Empirical antibiotic treatment should be modified according to the results of the Gram stain and cultures.
Clerc et al. recently published their hospital experience on septic arthritis at the University Hospital of Lausanne, Switzerland.28 The objective was to review the epidemiology of septic arthritis and establish local standards for empirical antibiotic therapy. This retrospective study was based on positive cultures of synovial fluid. Microbiological results and medical records were reviewed. Between 1999 and 2008, they identified 233 episodes of septic arthritis. S. aureus was the most common organism isolated (44.6%), followed by Streptococcus (14.2%). Only 11 cases (4.7%) of MRSA arthritis were diagnosed, of which 5 (45.5%) occurred in known carriers. Most patients had involvement of large joints, 147 (63%), of whom 40% had knee involvement. Involvement of small joints, especially hands and feet, was observed in 37% of cases. In patients with involvement of the small joints, most had a pre-existing impairment (65%) and most were diabetic (42%); the majority of patients had an adjoining infection, either osteomyelitis or soft tissue infection (94%). In comparison, hematogenous spread was the main form of infection in 76% of patients with involvement of the large joints. The authors conclude that amoxicillin-clavulanate or cefuroxime are adequate to empirically cover septic arthritis of the large joints. However, in diabetic patients with septic arthritis of the small joints, broad spectrum antibiotics would be significantly more effective. Systemic treatment of MRSA is not justified.
This study is representative of the region and may be applied to other areas or hospitals that share a similar epidemiology. Furthermore, studies have shown the absence of clear evidence regarding antibiotic selection or treatment duration. Epidemiology of the area and especially the local susceptibility patterns, need to be considered when initiating therapy with antibiotics in septic arthritis. The emergence of antimicrobial resistance, especially in certain geographic areas, makes the use and development of new antibiotics, alone or in combination, necessary. The introduction of new therapeutic modalities is under intense investigation, especially the use of cytokines, glucocorticoids and other antimicrobial peptides, either as adjuncts to antibiotic therapy or as antimicrobial agents.29–31
Reactive ArthritisReactive arthritis is classified within the group of spondyloarthritis, including ankylosing spondylitis, psoriatic arthritis, arthritis associated with inflammatory bowel disease, acute anterior uveitis, juvenile spondylitis, sacroiliitis and undifferentiated spondyloarthritis. The overall prevalence of this group of diseases is between 2% and 3%, which is at least 3 times more frequent than the prevalence of RA.32–34
It is considered that reactive arthritis is a disease that has been recognized since the time of Hippocrates, in the fourth century BC. However, the relationship between urinary tract infections and arthritis was first recognized in 1818 by Sir Benjamin Brodie, who described the triad of arthritis, urethritis and conjunctivitis in a group of patients. In addition, Brodie also recognized its recurrent nature, with exacerbations and remissions of the disease. In 1879, Neisseria gonorrhea was identified. Moreover, the association between arthritis and dysentery was established by Sydenham in the seventeenth century. However, this association between digestive tract infections and arthritis was clearly identified in 1916, at the end of the First World War, when several European authors, mainly from Germany and France, described several patients with diarrhea who subsequently, 3 to 4 weeks later developed arthritis, conjunctivitis and some, urethritis. The most important publications of this era were those by Fiessinger and Leroy in France and in Germany, Hans Conrad Reiter. In France, this disease still goes by the name Fiessinger and Leroy. However, in the English literature, the eponym of Reiter was established thanks to a publication by Walter Bauer and Ephraim Engleman, who published a series of patients with arthritis, conjunctivitis and urethritis and used a single reference, Reiter's. In the last 10 years, Reiter's nefarious past during World War II, which included human experimentation in Nazi concentration camps, has come to light, so many groups and prominent rheumatologists have requested that the disease be named reactive arthritis.35–38
At the end of the Second World War came a major natural experiment in the history of reactive arthritis. In 1944, Finland had an epidemic of Shigella dysentery that compromised over 150000 individuals, of whom 344 (<1%) developed arthritis and conjunctivitis, or reactive arthritis. These patients were followed through the years and most of them remained symptomatic, with a clinical spectrum that ranged from arthralgia, arthritis, ankylosing spondylitis to sacroiliitis. Furthermore, in the sixties the presence of histocompatibility antigen B-27 was determined and 90% were positive. This way, one can appreciate the natural history of the disease.39
Another way of classifying reactive arthritis according to the presence or absence of HLA-B27.40
The epidemiology of reactive arthritis is difficult to determine because of the lack of diagnostic criteria, because of the difficulty in identifying, recognizing and treating the causative organisms, which can alter the subsequent course of the disease, the genetic variability of HLA-B27 and the presence of local environmental factors that also play a role, such as Yersinia enterocolitica infection, which is more common in certain geographic areas than in others.40
The prevalence is about 0.1% in the general population, with an annual incidence of 10 cases per 100000 inhabitants. This represents a rather low estimate because there is no clear clinical difference, especially in the early stages, between subgroups of spondyloarthritis, since a large proportion of patients are asymptomatic, approximately 36% and 26% in Chlamydia enteral infection.40
Essentially, any infectious microorganism can result in reactive arthritis, but those more commonly involved are Chlamydia trachomatis (C. trachomatis), Yersinia, Salmonella, Campylobacter and Streptococcus (Table 2).
The pathogenesis of reactive arthritis is a complex, multifactorial, with involvement of the environment, genetic factors and the immune system. Environmental factors are represented by infectious agents, particularly gram-negative organisms. In humans, HLA-B27 plays a major role, although the exact mechanism of how it performs its role is unknown.41,42
The work of Taurog et al. in transgenic rats for HLA-B27 and human β2-microglobulin clearly established the importance of HLA-B27 in the pathogenensis.43,44 Most rats are normal at birth, but from the second and third week onward, begin to develop diarrhea, conjunctivitis, urethritis, arthritis of the peripheral and axial joints and lesions resembling skin psoriasis. The presence of other histocompatibility antigen does not induce an inflammatory process, which clearly establishes the HLA-B27 as a trigger of the process. Moreover, the participation of the environment was also clearly established with the second part of the study. If rats were maintained at birth in a sterile environment they did not develop the inflammatory process, indicating that the commensal flora is important in triggering the inflammatory process. These findings are indicative of the role of the commensal flora and are supported by the detection of commensal organisms by PCR in synovial fluid from patients with reactive44 arthritis. This is an area of intense research, as we will better appreciate when we discuss the involvement of infections in the pathogenesis of RA.
Reactive arthritis is a systemic disease that affects young people between the second and fourth decades of life. It can affect children and older individuals. It usually occurs 2–4 weeks after a genitourinary (male: female, 9:1) or enteric (male: female, 1:1) infection.40
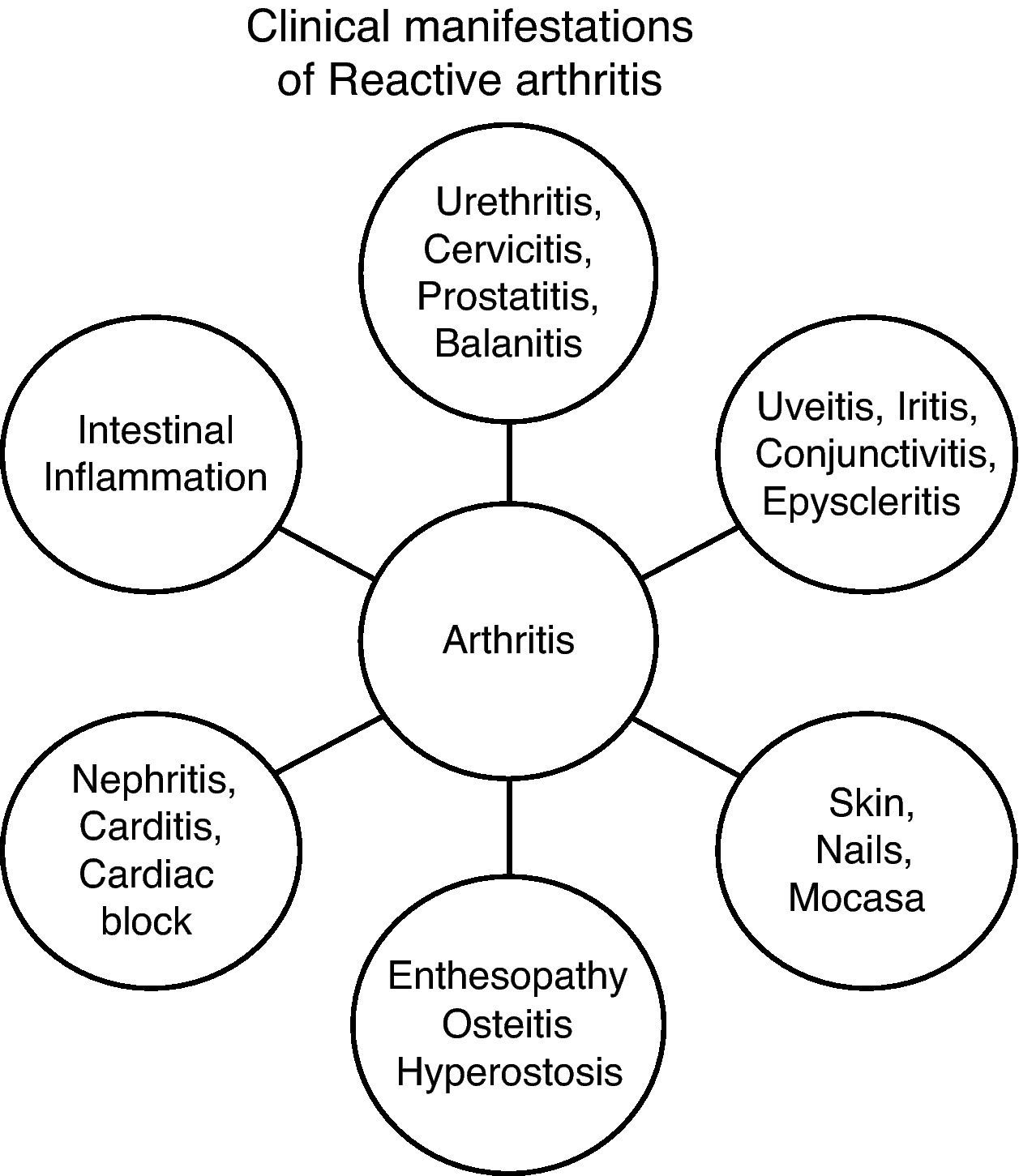
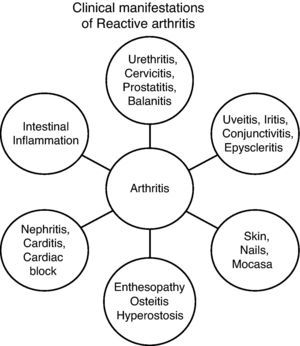
Clinical features associated with genitourinary infections are virtually identical to those associated with enteric infections. Clinically, there are 4 syndromes40 (Fig. 1):
- 1
Enthesopathic Syndrome.
- 2
Peripheral arthritis: acute or subacute asymmetric oligoarticular arthritis, which affects the lower limbs.
- 3
Axial and pelvic syndrome: spinal involvement with sacroiliitis.
- 4
Extramusculoskeletal Syndrome.
Laboratory results are completely nonspecific. The ESR and CRP are elevated in at least 50% of patients. The presence of HLA-B27 is found in about 60% of patients and in a lower percentage in non-Caucasian populations. P-ANCA positivity is detected in 20%–30% of patients, but this finding is not specific. Both synovial fluid and synovial membrane biopsies, as well as imaging, do not contribute much to the diagnosis.
The lack of validated diagnostic criteria represents a problem for the diagnosis of reactive arthritis. The criteria of the American College of Rheumatology require identification or documentation of urogenital infection by C. trachomatis or enteric infection. Patients who meet all requirements, except infection, are classified as undifferentiated monoarthritis or oligoarthritis. The classification of the European Spondyloarthritis Study Group are published, but not validated.45
The differential diagnosis includes other members of the spondyloarthritis, septic arthritis, Still's disease, Behçet disease and sarcoidosis group.
The prognosis is variable. Most patients remain symptomatic, with joint pain, back pain, ankylosing spondylitis and development of long-term (15–20 years) disease. Another group goes into permanent remission and a minority has a relapsing course. There are certain risk factors for poor prognosis: nature of the infection, persistent infection with Chlamydia, the presence of HLA-B27 (axial involvement, ocular), male gender, recurrent arthritis and a family history of the disease.
The vast majority of affected patients with reactive arthritis respond to treatment with nonsteroidal antiinflammatory drugs (NSAIDs), but a significant proportion requires treatment with a second line of disease modifying agents. Biological agents, especially TNF blockers, have great impact on the treatment of refractory reactive arthritis patients.46 Another advance in the management of reactive arthritis is evidence showing that the combined use of antibiotics may induce complete remission and cure to Chlamydia47 induced reactive arthritis.
It is well established in the literature that arthritogenic microorganism infection can present as an acute joint process and, in different proportions depending on the agent involved in the acute process, can originate a chronic process, with clinical variables, including a clinical spectrum of joint pain, recurrent or chronic arthritis, ankylosing spondylitis and frank sacroiliitis. On the other hand, and using the example of Chlamydia infection, evidence has shown that Chlamydia can be identified serologically and cultured in 62% of patients with chronic spondyloarthritis, including patients with reactive arthritis, psoriatic arthritis and ankylosing spondylitis.48 These findings suggested that Chlamydia infection occurs sometime during the course of the disease and support the use of antibiotics.47 Recently, these findings were corroborated in a group of patients with undifferentiated spondyloarthritis using a more sensitive technique to detect Chlamydia DNA by PCR in both peripheral blood and the synovial49 membrane. The findings demonstrated the presence of DNA of Chlamydia in the synovium of 62% of patients with undifferentiated spondyloarthritis, compared to patients with osteoarthritis (P<.0001).
These findings add to a series of studies showing persistent Chlamydia DNA detected by PCR in the synovium of patients with reactive arthritis.50–54 Furthermore, it has been shown that Chlamydia is metabolically active, morphologically distinct and has a different gene expression in persistent55 disease. In it, cellular proliferation genes such as genes for the outer membrane protein-1, are underexpressed, while genes of heat shock proteins are upregulated.56 The latter supports the notion that heat shock proteins (HSP) are of great importance in the persistence of Chlamydia. HSP allow surviving cells to prevent lethal attacks because of protein denaturation. HSP-60 plays a critical role in the inability of cells infected with Chlamydia to develop apoptosis. Therefore, it is quite likely that elimination of the Chlamydia HSP is important in preventing sequelae.57,58
The beneficial use of antibiotics in patients with arthritis, particularly those with intracellular action, has been reported since the early nineties.47 Since that time, there have been a number of studies, most of them open and uncontrolled, showing some benefit, especially in patients with reactive arthritis secondary to genitourinary tract infection.59–61
Furthermore, there is in vitro evidence supporting the use of certain antibiotics in Chlamydia. It has been shown that monotherapy with doxycycline or azithromycin for Chlamydia leads to an intracellular persistence stage, while the use of rifampicin induces resistance to infection by Chlamydia and also inhibits all transcripts, including the inhibition in general of HSP gene expression.62,63 Furthermore, it has been shown that the combination of azithromycin and rifampicin exerts a synergistic action capable of eradicating Chlamydia in cells of chronically infected patients with Chlamydia-induced arthritis, who experience significant improvement with the combination of rifampicin and doxycycline for 9 months, compared with doxicyclin64 monotherapy.
The evidence discussed supports the notion that the prolonged use of combinations of antibiotics and antimicrobial activity to inhibit the production capacity of the Chlamydia HSP-60 could cure chronic reactive arthritis induced by Chlamydia, somewhat similar to what happens with other persistent intracellular organisms, such as Mycoplasma tuberculosis. This was the basis for the prospective, controlled use of antibiotic combination therapy in patients with chronic reactive arthritis induced by Chlamydia.65
The main objective of the study was to investigate whether a 6-month course of combination antibiotics was effective in patients with reactive arthritis induced by chronic Chlamydia infection. This study lasted 9-months and was prospective. Eligible patients had to be positive for C. trachomatis or C. pneumoniae by PCR. The groups received: (a) doxycycline and rifampicin plus placebo instead of azithromycin, (b) azithromycin and rifampicin plus placebo in place of doxycycline, or (c) placebo instead of azithromycin, doxycycline and rifampicin. The primary end point was the number of patients who improved 20% or more in at least 4 of 6 variables, no worsening in any variable in both groups with combined antibiotic and the placebo group at 6 months compared with baseline. The primary endpoint was achieved in 17 of 27 patients (63%) receiving combination antibiotics and in 3 of 15 patients (20%) receiving placebo. Secondary objectives showed similar efficacy. Six of 27 patients (22%) receiving combination antibiotics had their illness resolved completely, whereas no patients given placebo achieved remission. More patients in the active treatment group became significantly negative for C. trachomatis or C. pneumoniae by PCR at 6 months. Adverse reactions were mild, with no difference between groups. It was concluded that a 6-month course of combination antibiotics is an effective treatment for chronic reactive arthritis induced by Chlamydia.65
It is important to reproduce these results in an early reactive arthritis study as well as in reactive arthritis induced by other germs.
Arthritis Associated With Infection by the Human Immunodeficiency VirusThe HIV pandemic originated more than three decades ago and the association between HIV and arthritis was recognized in the early eighties.66–68 The clinical spectrum and frequency of inflammatory joint manifestations associated with HIV have changed over the years, especially with the introduction of HAART in the nineties.69,70 Arthritis and other inflammatory joint manifestations of this nature continue to occur in association with HIV, but the frequency is lower and the types of clinical manifestations have also evolved.
HIV-associated rheumatic manifestations may be observed in any stage of HIV infection, but in the West they tend to be more common in advanced stages III and IV, while in Africa and other developing countries they are generally seen in Stage I and II. It is not uncommon in the latter for arthritis or other inflammatory musculoskeletal manifestations to be related to HIV.71,72
The pathogenesis of arthritis and other inflammatory manifestations associated with HIV infection is not clearly established, is quite complex and multifactorial, and has some factors related to the environment (bacterial infections) and genetic factors (HLA-B27 and MICA) as well as some related to the innate immune response and cell mediated immunity.73–78
Inflammatory joint manifestations before HAART may be seen in Table 3.79–85
Joint Manifestations Associated With Human Immunodeficiency Virus: Before and After Highly Active Antiretroviral Therapy.
| Before HAART |
| Joint pain |
| Arthritis |
| Reactive arthritis |
| Psoriatic arthritis |
| Undifferentiated spondyloarthropathy |
| DILS |
| Myopathy-polymyositis, dermatomyositis, inclusion body myositis |
| Vasculitis |
| Gout |
| Infections: Septic arthritis, osteomyelitis, pyomyositis |
| After HAART |
| Immunologic reconstitution inflammatory syndrome: sarcoidosis, lupus, rheumatoid arthritis, DILS |
| Drug induced conditions |
| Zidovudine myopathy |
| Nemaline bars |
| Rhabdomyolysis |
| Gout and hyperuricemia |
| Lipodystrophy |
| Metabolic and infectious conditions |
| Osteoporosis |
| Avascular necrosis |
| Osteomyelitis |
| Pyomyositis |
| Septic arthritis |
DILS, diffuse infiltrative lymphocytosis syndrome; HAART, highly active antiretroviral therapy.
Joint pain was the most common manifestation, both as part of the acute viral syndrome and in any stage of the disease. Its duration is short, rarely more than 2 weeks, and involves the knees, shoulders and elbows more frequently. The response to analgesics and NSAIDs is pretty good.
Acute articular syndrome is characteristic of intermittent painful advanced stages of HIV and is short but with a poor prognosis. The use of opioids is necessary for its control.
HIV-associated arthritis can occur at any stage of the disease and is usually of short duration (no more than 6 weeks) affecting 3–4 large joints of the lower limbs and is non erosive. Usually, there is no effusion, but when It occurs it is located especially at the knees and the synovial fluid is non inflammatory, with negative cultures, with the presence of tubuloreticular inclusions. The P-24 antigen of HIV has been detected in the synovial tissue, as have CD4 and CD8.86 HIV-associated arthritis is usually self-limiting and responds well to NSAIDs or low-dose prednisone. It occasionally, requires the use of hydroxychloroquine and sulfasalazine. The presence of Jaccoud arthropathy has been described occasionally.87
Spondyloarthritis, including reactive arthritis, psoriatic arthritis, ankylosing spondylitis and undifferentiated spondyloarthritis have been associated with HIV infection. These forms of arthritis were first reported to initially constitute about 30 to 40% of inflammatory joint manifestations associated with HIV. In Caucasians, they are strongly associated with HLA-B27, but in others popuations, particularly in Central Africa, they are quite common and are not associated with HLA-B27. Further, Central Africa, before HIV, did not present practically any form of spondyloarthritis, although bacterial infections are quite prevalent.88 Extraarticular manifestations of spondyloarthritis, particularly skin manifestations, are present. The route of contamination seems to influence the clinical expression of HIV-associated spondyloarthritis, which is less frequently observed in individuals who use intravenous drugs. Most patients respond to NSAIDs, although a large percentage of patients will need treatment with methotrexate, hydroxychloroquine or low doses of glucocorticoids. Patients with axial compromise and those with severe forms of psoriatic arthritis benefit from the use of biological agents, especially TNF inhibitors.89
The diffuse infiltrative lymphocytosis syndrome (DILS) is characterized by the presence of massive growth of the parotid and lacrimal glands, occurs bilaterally and painlessly and presents peripheral CD8lymphocytosis.90 Often confused with Sjögren's syndrome, the absence of anti SS-A and SS-B and the presence of extraglandular manifestations, such as lymphocytic interstitial pneumonia, which occurs in 30-50%, and facial paralysis, which occurs in 30% of patients with HIV, helps differential diagnosis.91 There is some geographic distribution for this syndrome, being more common in Africa than in the U.S. Treatment is usually symptomatic, and high doses of glucocorticoids and HAART are required.
Inflammatory muscle involvement is relatively common in HIV-infected patients. Myalgia, polymyositis, dermatomyositis, inclusion body myositis, nemaline myopathy, rhabdomyolysis and sarcopenia may be observed during the course of the disease. Polymyositis is the most common syndrome, usually occuring in the early stages of infection, but can occur at any stage. It is an inflammatory process associated with elevated muscle enzymes, especially creatine, CD8 cell infiltration, and identification of HIV antigens. The clinical course and response to treatment is very similar to that of idiopathic myopathy. Sarcopenia is usually seen as part of the AIDS complex, and muscle atrophy is accompanied by severe loss of weight, diarrhea, weakness and fever. It is currently very rarely observed.
Fibromyalgia is common in HIV patients is associated with depression and therapy is similar to that of uninfected patients.
Vasculitis that involves small, medium and large caliber vessels can be seen in HIV infection. It can occur at any stage of the disease, usually in young people and a wide clinical spectrum can be present.92
Infections, including septic arthritis, pyomyositis and osteomyelitis, can be observed in patients with HIV, especially with opportunistic infections; the musculoskeletal system usually is affected in patients with severe immunosuppression, with CD4 lymphocyte counts below 200cells/mm3. The clinical behavior and therapeutic response are very similar to those occurring in infected patients.93
Hyperuricemia and gout are highly prevalent in patients with HIV, and their presence may have prognostic implications.
Rheumatic processes that are seen in the era of HAART are summarized in Table 3.
HAART has revolutionized the natural history, morbidity and mortality of HIV-infected populations, and has also positively influenced the frequency and clinical expression of associated inflammatory joint manifestations. At present, at least in Western countries, there is still spondyloarthritis, but the frequency is decreasing. The presence of opportunistic infections and neoplastic complications is higher in certain geographic regions including ours and frequency of DILS is reported to be lower by some researchers.94 However, new syndromes, especially the immune reconstitution inflammatory syndrome (IRIS), and musculoskeletal complications directly related to the use of HAART have been recognized.93
IRIS is a systemic inflammatory process which occurs in patients with HIV after initiation of HAART and is characterized by an elevation of CD4 cells, an increased CD4+/CD8+ count and elevation of proinflammatory cytokines circulating such as IL-6 and interferon gamma. Other immunologic abnormalities include Th1/Th2 imbalances and increased expression of CCR-3 and CCR-5 in monocytes and granulocytes. Most cases of IRIS occur 3–24 months after initiation of HAART and may present as a number of syndromes resembling autoimmune diseases such as lupus, sarcoidosis or Graves93 disease.
Zidovudine-induced myopathy was one of the first complications of HIV therapy. It is accompanied by myalgia, pain and proximal muscle weakness, and usually occurs after the first year of therapy. It appears to be secondary to mitochondrial dysfunction and the clinical and muscle enzyme elevations disappear after zidovudine is discontinued. The use of protease inhibitors may induce severe cases of muscle involvement, including rhabdomyolysis, especially when used in combination with statins.
A series of soft tissue complications, including adhesive capsulitis, Dupuytren's contracture, tenosynovitis and temporomandibular joint dysfunction, have been reported with the use of protease inhibitors.
Metabolic complications such as insulin resistance, dyslipidemia, hyperuricemia and gout, and metabolic bone disorders, including osteoporosis and osteopenia, osteomalacia and, potentially, osteonecrosis have been described in association with HAART. The exact pathogenesis of these associations is not clearly elucidated, but HAART is involved to some extent.95
The Infectious Etiology of Rheumatoid ArthritisThe cause of RA is not well understood. Smoking is a strong environmental risk factor as well as alcohol consumption, antioxidant intake and environmental pollution which may also play a role. An infectious cause for RA has been postulated, but to date no organism has been convincingly implicated. Since the last century, the theory that the disease depends on an infection of some kind has been proposed as a reasonable hypothesis. This should not be interpreted as the presence of a joint infection per se. This situation may occur, but in most cases of arthritis it appears to be dependent on a local infection somewhere in the body, absorption and spreading of inflammatory mediators to the joint. There are certain characteristics in RA that suggest the presence of an infection, such as the characteristics of the attack, which often suggest an acute infection, and the presence of certain complications, such as pericarditis and pleural effusion. Two sites have long been considered as potential sources of infection, the intestine, which could lead to the entry of microorganisms, which would not necessarily be pathogenic under ordinary conditions and the mouth, which may be another foci of microorganisms, which could be ingested continuously and eventually would lead to an inflammatory process. This view suggests that arthritis is secondary to an infection of some sort, probably with several agents involved, and the end result would lead to joint inflammation. This hypothesis, suggested by Osler and McCrae over 100 years ago, was shared by many other researchers of their time.96
Several recent studies support the infectious cause of RA. Rosenau and Schur recently published evidence suggesting that the measles virus could be the responsible.97 The aim of the study was to identify a virus in the rheumatoid synovium. Tissue was obtained during knee replacements surgery and tested for virus (cytopathic effects). Concurrently, sera from 50 patients with rheumatoid factor were tested for IgM antibodies to the viral agent. The results showed that the measles virus was identified and 11 of 50 (22%) of the sera positive for IgM antibodies. The authors concluded that there is an association between measles virus and RA.97 Another group of authors investigated the importance of characteristics at birth and the presence of infections early in life, at the risk of RA and juvenile idiopathic arthritis. The results showed that infections contracted during the first year of life were associated with an increased risk of developing seronegative arthritis, but HIV positive patients do not develop arthritis or juvenile idiopathic arthritis. The conclusion was that infections during the first year of life, and possibly also factors related to fetal growth and at the time of birth, can be important in cases of RA and juvenile98 idiopathic arthritis.
Bacterial and viral components are an attractive source of antigens capable of inducing RA. Streptococcal mycobacterial antigens and are also good inducers of experimental arthritis. Rubella, parvovirus B19 and arboviruses can cause arthritis in humans. Using RT-PCR, studies have shown a plethora of bacterial rRNA sequences in the synovial membrane, a finding that is unlikely to be caused by contamination. However, no organism has been clearly identified and associated with RA. The presence of these microbial antigens correlates better with inflammation that diagnosis. The normal synovial membrane contains no bacterial rRNA and it is quite likely that the inflamed synovium is colonized by bacteria, especially bowel and skin commensals. The joints in RA remain sterile by conventional culture.
The inflamed synovium colonization by bacteria and bacterial products is relatively common and may contribute to the pathogenesis of RA. Bacteria and their products (LPS, bacterial DNA and peptidoglycans) are potent activators of the innate immune system and act as adjuvants, permitting T and B cell adaptive responses. “Toll”-Like receptors, especially TLR, recognize bacterial DNA and may be important in the induction of arthritis in TNF dependent animal models. Furthermore, activation of antigen presenting cells such as dendritic cells, bacterial DNA and other ligands of “Toll” receptors may allow the generation of a response to autoantigens within the joint.
Of great interest, with respect to the infectious cause of RA, is the relationship between periodontitis, Porphyromonas gingivalis (P. gingivalis), anti-CCP antibody (anti-CCP), and disease.99–106 The prevalence of periodontal disease is increased 2-fold in patients with RA, which is not due to the presence of secondary Sjögren's syndrome. Periodontitis and RA share pathogenic mechanisms, environmental risk factors, such as smoking, and associated epidemiological107 studies. An increased prevalence of the shared epitope HLA-DRB1 * 0104, increased T cell responses with high tissue levels of IL-17 and exaggerated responses of B cells with a predominance of plasma cells in the affected gingival tissue exist in both entities.103,108 Furthermore, DNA of P. gingivalis is commonly found in the rheumatoid synovium and antibody levels against the bacteria are higher in patients with RA, which have positive anti-CCP antibodies. These findings suggest that periodontitis may contribute to the pathogenesis of RA.
P. gingivalis is a common cause of periodontitis and has been proposed as the etiological link between RA and periodontitis.103 In addition, P. gingivalis possesses proteolytic enzymes (gingipains) responsible for the virulence of the microorganism.109P. gingivalis is the only prokaryotic organism that expresses the enzyme peptidylarginine deiminase (PAD), which converts arginine into citrulline in normal105 tissues. The bacterial protein citrullination by PAD is a property of P. gingivalis and is not present in other oral commensal organisms. Accumulating evidence suggests that the presence of autoimmunity to citrullinated proteins is specific to the pathogenesis of RA. Citrullination, also referred to as deamination, is a modification of the side chains of arginine, catalyzed by PAD. This posttranslational modification has the potential to alter the structure, function and antigenicity of the protein fibrinogen, vimentin, collagen type II and alpha-enolase. All these proteins are expressed in the joint. The antibodies to fibrinogen and collagen type II promote inflammation through immune105 complex formation. Antibodies to citrullinated proteins are associated with shared epitope alleles and autoimmunity to the CEP-1 peptide of citrullinated alpha-enolase and shows association with smoking and HLA-DRB1 * 0401, * 0404, and PTPN22 620W.
It may be concluded that there is evidence supporting the relationship between periodontal disease and the development of RA. Periodontal pathogens have direct access to the systemic circulation. PAD represents an important pathogenic factor for RA. Currently, P. gingivalis is the only bacterium expressing the enzyme and PAD plays a role in the humoral immune response and in the presence of anti-CCP antibodies in patients with RA. Oral hygiene and smoking represent environmental factors that are able to influence the risk for the development of RA. The evidence discussed is of considerable interest and importance, but there is a need for more clinical, especially longitudinal studies, to establish a clear and defined relationship between RA and periodontal disease.
The other aspect to be discussed is the relationship between intestinal bacteria and RA. These studies have been possible using DNA technology, while bacterial cultures are difficult and tedious. Researchers are using DNA sequences to identify all bacteria present in the mouth and intestine of study participants. Scher and Abramson to date have studied 90 patients, 55 adults with RA and 35 controls.110 Regarding the oral microbiota, the findings show that patients with early RA are 3 to 4 times more likely to have P. gingivalis than healthy controls. In general, periodontal disease was present in 82% of chronic RA patients and in 75% of patients with early RA. Regarding the intestinal microbiota, intestinal bacteria associated with inflammation were more prevalent in RA patients than in healthy controls. Prevotellaceae species was identified in approximately 80% of RA patients compared with the 20% typically found in healthy controls. These preliminary findings support the data from previous studies and show a high prevalence of oral disease in patients with RA. This study is underway, and the authors are contemplating the use of antibiotics to modify the microflora of the body and establish how the bacterium causes inflammation. The findings could lead to development of strategies to reduce or prevent inflammation before the triggering of RA.
In conclusion, the relationship between microbial germs and joints is quite common and complex. The clinical spectrum observed is broad and ranges from septic arthritis, which demonstrates the presence of the organism in the joint, to systemic diseases such as RA, in which, using sensitive technology, a pathogenic role of commensal bacteria may be explained. This review does not include certain viral infections that are emerging in the Western world, such as arthritis induced by the alpha virus Chikungunya111 or joint disease studied with PCR technology, such as Whipple's112 disease. Furthermore, it is noteworthy that biological therapy is not associated with an increased prevalence of septic arthritis.113 It is obvious that the relationship between infection and arthritis is still valid and we must remain alert to the possible emergence of new pathogens and complications associated with new therapies under use in rheumatology.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Espinoza LR, García-Valladares I. Microbios y articulaciones: la relación entre infección y articulaciones. Reumatol Clin. 2013;9:229–38.