To assess health-related quality of life (HRQoL) and oral health-related quality of life, and correlate them with unstimulated whole salivary flow (UWSF) and oral sicca symptoms in patients with primary Sjögren's syndrome (PSS).
MethodsWe included 60 patients with PSS and 60 healthy controls matched according to gender and age (±3 years). We measured the UWSF and scored the European League Against Rheumatism (EULAR) Sjögren's Syndrome Patient Reported Index (ESSPRI). We assessed the short version of the SF-36 as a generic measurement of HRQoL and the Xerostomia Quality of Life Scale (XeQoLS) questionnaire to evaluate oral quality of life. We evaluated oral symptoms using an 8-item Visual Analogue Scale (VAS) questionnaire.
ResultsWe observed a poorer HRQoL (lower scores in SF-36) and oral quality of life (higher scores in XeQoLS), as well as a greater severity of symptoms in the VAS questionnaire upon comparing patients vs controls. The XeQoL correlated with the UWSF (τ=−0.24, P=.008), the ESSPRI (τ=0.45, P=.0001), VAS 1–2 and VAS 5–8 and the SF-36 score (τ=−0.28, P=.002).
ConclusionsPatients with PSS had a poorer HRQoL and oral quality of life than controls. UWSF contributes to the oral quality of life which, in turn, has an impact on HRQoL. Symptomatic treatment of xerostomia as well as the prevention of infections, decay and tooth loss would help to improve the oral quality of life in these patients.
Evaluar la calidad de vida general y oral, y sus correlaciones con el flujo salival no estimulado (FSNE) y los síntomas de xerostomía en pacientes con síndrome de Sjögren primario (SSP).
MétodosSe incluyeron 60 pacientes con SSP y 60 controles pareados por género y ±3 años de edad. Se midió el FSNE y se aplicó cuestionario ESSPRI (Cuestionario reportado de síntomas en Síndrome de Sjögren de la Liga Europea contra el reumatismo [EULAR]). Se utilizó la versión corta de SF-36 para evaluar calidad de vida general, y para la calidad de vida oral el cuestionario XeQoLS; así como 8 preguntas para evaluar síntomas orales (dificultad al hablar, tragar, cantidad de saliva en boca, sequedad de boca, garganta, labios, lengua y nivel de sed) mediante escalas visuales análogas (EVA).
ResultadosObservamos peor calidad de vida general (menor puntuación SF-36), oral (mayor puntuación XeQoL) y mayor sintomatología evaluada por EVA en pacientes vs controles. El XeQoL correlacionó con el FSNE (τ=−0,24, p=0,008), el ESSPRI (τ=0,45, p=0,0001), las preguntas EVA 1-2 y EVA 5-8 y la escala SF-36 (τ=0,28, p=0,002).
ConclusionesLos pacientes con SSP tienen peor calidad de vida general y oral que sujetos sanos. El FSNE contribuye en la calidad de vida oral y a su vez la calidad de vida oral impacta en la calidad de vida general. La intervención oportuna de terapia sintomática de xerostomía y la prevención de infecciones, caries y pérdida dental pudiera impactar la calidad de vida de estos pacientes.
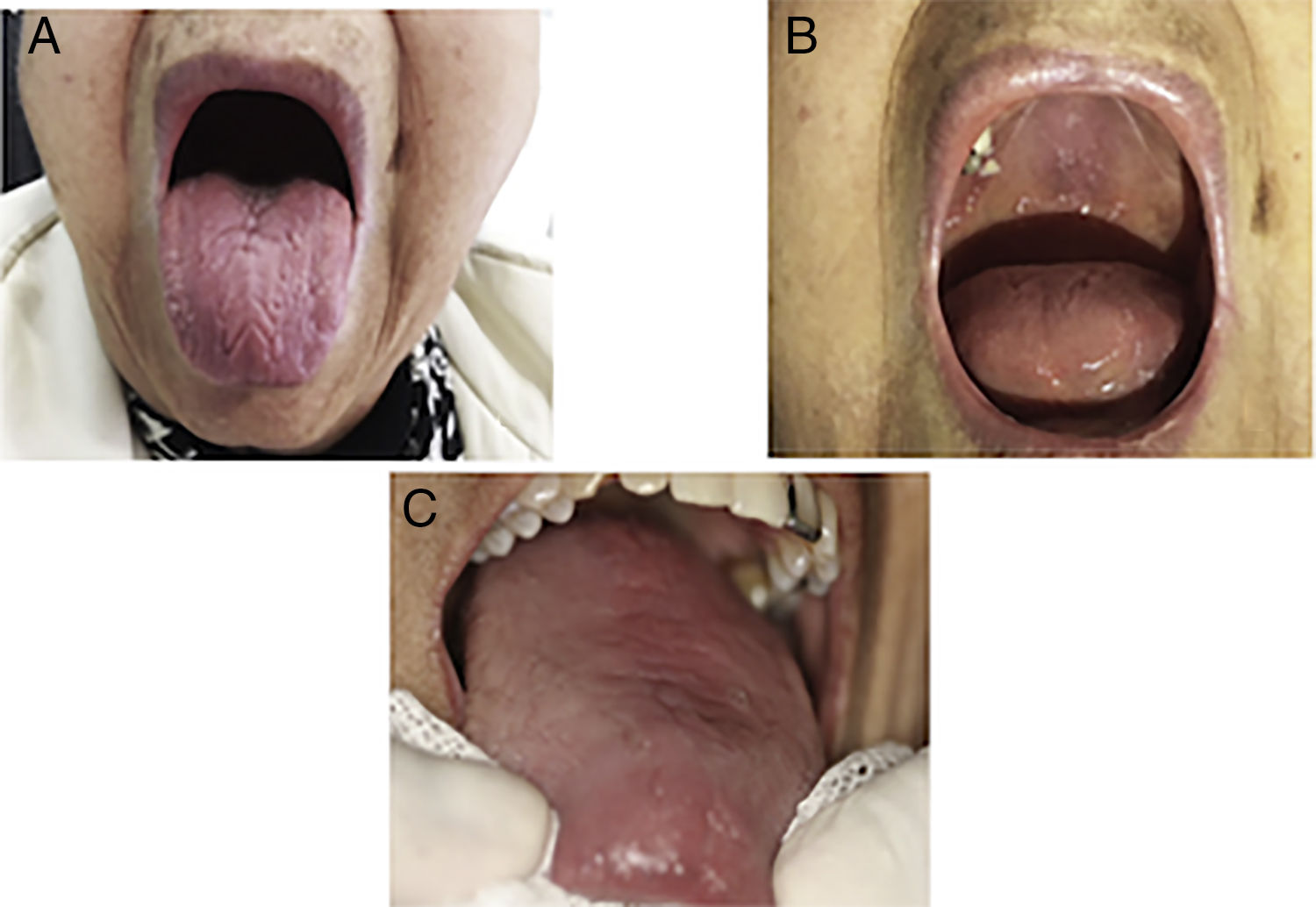
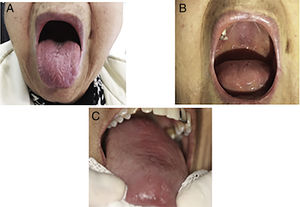
Primary Sjögren's syndrome (PSS) is a chronic autoimmune disease characterized by oral sicca symptoms and extra glandular features. The oral symptoms are the features of the greatest discomfort in patients with Sjörgen's syndrome. Xerostomy is the subjective sensation the patient perceives of a scarcity or absolute lack of saliva in the oral cavity. It is an unpleasant symptom and also hinders the ability to talk, chew and swallow. Low saliva flow is a risk factor for the development of tooth decay, loss of teeth, oral infections and problems with prosthetic components1 (Fig. 1).
Previous studies in PSS have reported a reduction in the overall quality of life of these patients compared with healthy control groups2–4 but a similar quality of life to patients with xerostomy from other aetiologies.5,6 It has also been observed that the quality of life in PSS is influenced by different factors, the most frequent being pain and fatigue, xerostomy7 and the presence of depression or anxiety.8
With regard to oral health-related quality of life in patients with PSS, little information exists. Two previous studies reported that xerostomy has a negative effect on oral quality of life in this patient group.9,10
The aim of this study was to assess general quality of life and oral quality of life and correlate it with unstimulated whole salivary flow (UWSF) and the EPSSRI in a group of patients with PSS.
MethodsThis was a cross-sectional study which included consecutive patients with a diagnosis of PSS who regularly attended the INCMNSZ Rheumatology Department, a tertiary level healthcare centre during the period from September 2016 to January 2017. All the patients fulfilled the ACR/EULAR11 criteria and patients with other concomitant autoimmune diseases were excluded. A randomized control group was also included of individuals without any autoimmune diseases or diabetes matched according to gender and age±3 years to the patients, who came from a Dental Health Unit for the general population during the same time period. The controls were selected based on the absence of xerostomy.
The patients were interviewed in person by a rheumatologist and a dentist who conducted a standard interview for the obtainment of demographic and clinical data. Participants were requested not to have eaten or drunk anything, consumed gum or had any oral hygiene procedure for at least 1h previously. The study took place in a closed room without any air-conditioning between 8a.m. and 11a.m. The unstimulated saliva flow was assessed by the same examiner. With the subject seated, they were asked to rest for 5min prior to the test, to minimize orofacial movements and not to speak. Prior to starting the procedure the patient swallowed residual saliva and were then requested to accumulate all the saliva in the floor of the mouth and expulse it into a graduated tube each minute. The saliva was collected for a period of 15min and the volume was measured by expressing it in ml/5min. A flow of ≤1.5ml/15min12 was considered abnormal.
Assessment of general and oral quality of lifeGeneral quality of life was classified using the SF-36 short version13 questionnaire scale of the version validated into Spanish. This tool comprises 36 questions (8 domains): physical function, physical role, body pain, general health, vitality, social function, emotional role and mental health. This in turn may be summarized into two components: physical (PCS) and mental (MCS). The higher the score the better the quality of life.
In the absence of a specific oral quality of life questionnaire in SS, we used the quality of life questionnaire in xerostomy XeQoLS.14 This tool comprises 15 questions which assess: physical function, pain, personal/psychological function and social function. The higher the score the poorer the quality of life.
Oral symptomsThe EPSSRI questionnaire was applied which was a tool validated in PSS for assessment of dryness and pain symptoms (the higher the score the worse the symptom).15
Eight questions from the visual analogue scale (VAS) were also applied which assessed difficulty in speaking, difficulty in swallowing, amount of saliva in the mouth, dryness of mouth, dryness in the throat, dryness of lips, dryness of tongue and level of thirst16 (Table 1). The level of discomfort in each question is transferred to a line of 100mm, interpreting that the higher the score the higher the discomfort, with the exception of question 3 where the higher the score the greater the presence of saliva.
Symptoms assessed by visual analogous scale: they assess the degree of discomfort from dryness, amount of saliva and degree of thirst.
| Assess the difficulty they feel when talking due to dryness in the mouth |
| Assess the difficulty they feel on swallowing due to dryness in the mouth |
| Assess how much saliva they have in their mouth |
| Assess dryness in the mouth |
| Assess dryness in the throat |
| Assess dryness in the lips |
| Assess dryness on the tongue |
| Assess level of thirst |
The study was approved by the institutional Ethics Committee and all the participants signed an informed consent form.
Statistical analysisDescriptive statistics was used. The categorical variables were compared using the X2 test and the numerical variables with the Student's t-test or the Mann–Whitney U test in accordance with the normality of each variable. The non-parametric Kendall's tau correlation coefficient was used. The value of P which was considered significant was <.05 two-tailed. The statistical software SPSS 20.SPSS was used for data analysis.
ResultsSixty patients with primary SS and 60 control subjects were included. The majority of the participants were women, 93.3% in the PSS group and 96.6% in the control group (P=.85). The average age was 55.5±14.3 years in patients and 55.7±8.1 years in the control group (P=.99). The patients had an average of 7.6±4 years from disease onset.
The patient group with PSS presented with xerostomy at 96.6% (n=58), xerophthalmia 95% (n=57), anti-Ro antibodies/SSA 63.3% (n=38), anti-La/SSB 41.6% (n=25), fluorescein staining 68.8% (n=31/45), salivary gland biopsy 92% (n=46). Twenty eight patients (46.6%) reported partial or total tooth losses. As expected UWSF was lower in the patients than in the control group, .25ml/15min (range 0–4) and 1.8ml/15min (range .7–3.2), P=.0001, respectively.
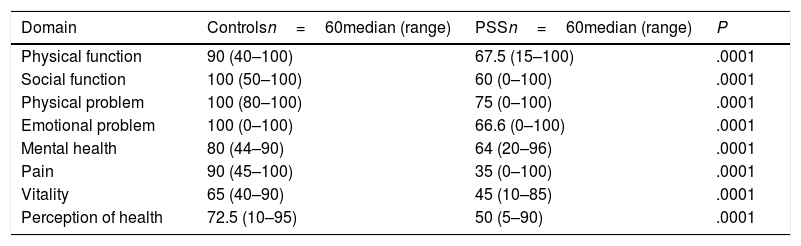
Quality of life and symptomsThe SF-36 questionnaire score of the patients with PSS was 61.5 (range 17–93) and that of the controls was 85.9 (range 54.3–96.3), P=.0001. We also found there was a statistically significant difference between the physical component (50.5 vs 82.5, P=.0001) and the emotional one (66.3 vs 91.4, P=.0001) between patients and controls. The score of each of the domains is contained in Table 2.
Results of the SF-36.
| Domain | Controlsn=60median (range) | PSSn=60median (range) | P |
|---|---|---|---|
| Physical function | 90 (40–100) | 67.5 (15–100) | .0001 |
| Social function | 100 (50–100) | 60 (0–100) | .0001 |
| Physical problem | 100 (80–100) | 75 (0–100) | .0001 |
| Emotional problem | 100 (0–100) | 66.6 (0–100) | .0001 |
| Mental health | 80 (44–90) | 64 (20–96) | .0001 |
| Pain | 90 (45–100) | 35 (0–100) | .0001 |
| Vitality | 65 (40–90) | 45 (10–85) | .0001 |
| Perception of health | 72.5 (10–95) | 50 (5–90) | .0001 |
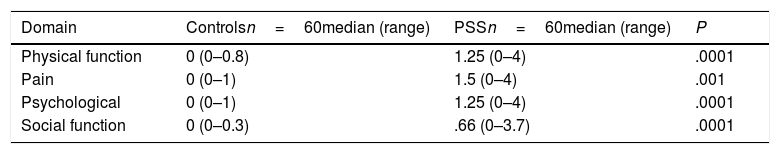
The total score of the XeQoL questionnaire was higher in patients with PSS than controls: 1.13 (0–3.8) vs 0 (0–0.6), P=.0001), indicating worse quality of oral life in patients. The results of each of the domains of the tool are shown in Table 3.
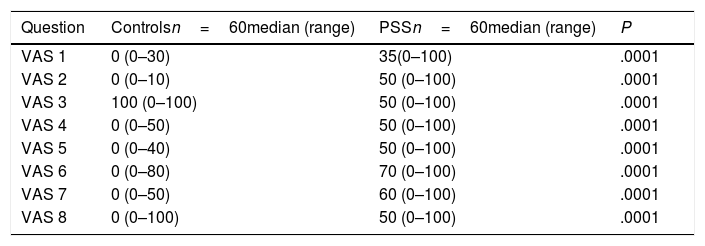
The mean score of the EPSSRI questionnaire in patients was 7.2±2.6. Table 4 shows the results of the assessment of the 8 VAS questions. We find there are significant differences between patients and controls showing poor results in the patients with PSS.
Results of the VAS questionnaire.
| Question | Controlsn=60median (range) | PSSn=60median (range) | P |
|---|---|---|---|
| VAS 1 | 0 (0–30) | 35(0–100) | .0001 |
| VAS 2 | 0 (0–10) | 50 (0–100) | .0001 |
| VAS 3 | 100 (0–100) | 50 (0–100) | .0001 |
| VAS 4 | 0 (0–50) | 50 (0–100) | .0001 |
| VAS 5 | 0 (0–40) | 50 (0–100) | .0001 |
| VAS 6 | 0 (0–80) | 70 (0–100) | .0001 |
| VAS 7 | 0 (0–50) | 60 (0–100) | .0001 |
| VAS 8 | 0 (0–100) | 50 (0–100) | .0001 |
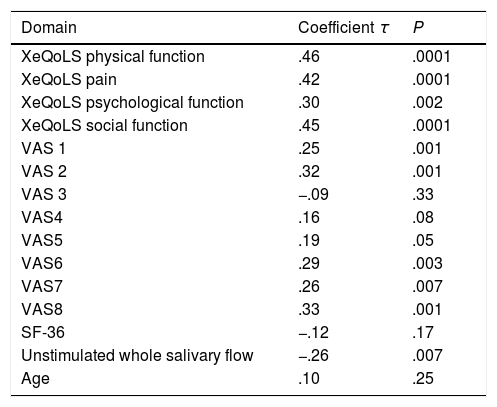
The EPSSRI score correlated negatively with the UWSF (τ=−.26, P=.007) and positively with the XeQoL (τ=.54, P=.0001) as well as each of its components and the questions 5, 6, 7 and 8 of the VAS (Table 5). No correlation was observed with questionnaire SF-36, or any of its domains, or with age.
Correlations with EPSSR.
| Domain | Coefficient τ | P |
|---|---|---|
| XeQoLS physical function | .46 | .0001 |
| XeQoLS pain | .42 | .0001 |
| XeQoLS psychological function | .30 | .002 |
| XeQoLS social function | .45 | .0001 |
| VAS 1 | .25 | .001 |
| VAS 2 | .32 | .001 |
| VAS 3 | −.09 | .33 |
| VAS4 | .16 | .08 |
| VAS5 | .19 | .05 |
| VAS6 | .29 | .003 |
| VAS7 | .26 | .007 |
| VAS8 | .33 | .001 |
| SF-36 | −.12 | .17 |
| Unstimulated whole salivary flow | −.26 | .007 |
| Age | .10 | .25 |
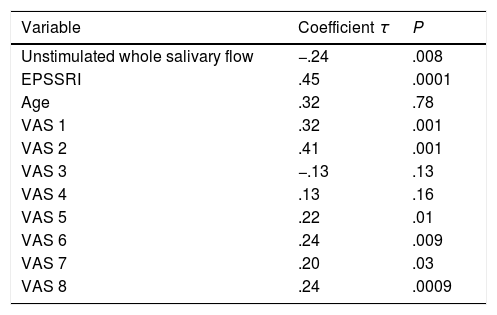
The XeQoL questionnaire correlated negatively with the UWSF and positively with the EPSSRI, VAS1 and VAS 5–8. Table 6 contains the results. The SF-36 did not correlate with any variables except that of the XeQoLS τ=.28, P=.002.
The UWSF correlated negatively and was statistically significant (P=.00001) with all the symptoms assessed by the VAS questions (VAS1 τ=−.51, VAS2 τ=−.54, VAS4 τ=−.50, VAS5 τ=−.44, VAS6 τ=−.38, VAS7 τ=−.5 and VAS8 τ=−.20), with the exception of number 3 where the correlation was positive (VAS3 τ=.23, P=.00001).
DiscussionHealth-related quality of life is a concept which refers to individual perception of mental and physical status and has been used to measure the effects of chronic diseases in several groups of patients to understand how diseases interfere in daily life. Greater awareness would lead to the identification of specific strategies to improve it.17
To date there is some information about general quality of life in patients with PSS. Existing studies have shown that the presence of this disease has a negative impact, in other words the quality of life is worse than in the control groups.2–4 For example, in one study conducted in the general population in United Kingdom, a poorer quality of life was observed in patients with PSS, on assessing it through the EQ-5D questionnaire. The most affected components were the domains of pain and depression.18 Equally, Inal et al. In their study assessed the quality of life in patients with PSS using the SF-36, WHOQOL-BREF and HADS questionnaires, and also found a poorer quality of life in patients with PSS vs healthy subjects.19
In our study we also reported a poorer quality of life in our patients on comparing this with controls matched by age and gender, assessed by the SF-36 questionnaire. As in other studies, the patients with PSS scored lower than the controls in all dimensions of the SF-36. We observed that the dimension of pain was the domain most affected, followed by vitality. In contrast, in the study by Inal et al., the domain vitality was the only one with a better score in patients with PSS compared to the control group. This finding which could have been contradictory, was described by the author as a consequence of an adaptation to health changes and to social support.19 However, in our study the least affected domain was the one which assessed physical problems (degree to which health impairments interfered in work and other daily activities) which was the most affected in patients with PSS in the American population.4
In their study Lendrem et al. used a questionnaire of 5 dimensions (EQ-5D), found that the higher values of the ESSDAI (activity in PSS index) and the EPSSRI index (assessment of dryness and pain symptoms) were associated with a worse quality of life.20 Equally, a study of patients with PSS in the Korean population reported that patients with a lower quality of life assessed by the SF-36 had higher scores of the EPSSRI.21 In our study we did not corroborate this correlation.
Furthermore, the reduction in saliva production in these patients predisposes them to mucous membrane infections, recurrent dental decay, loss of teeth, and loss of the ability to speak, eat and swallow. In their study Rusthen et al., assessed 31 patients with PSS and 33 controls (without sicca symptoms) using the tool the Oral Health Impact Profile (OHIP-14) and they observed a poorer oral quality of life in the patients, which was correlated with dysgeusia, sensation of burning on the tongue and halitosis.22 Equally, in another study, using the same questionnaire in its version of 49 questions (OHIP-29) they also reported a lower quality of life in patients with SS (primary or secondary) vs Controls in all their domains with the exception of pain.23 In contrast another study did not show differences in the OHIP-29 scoring between patients with PSS and controls.24 In our study we observed a lower quality of oral health assessed using the XeQoL questionnaire in patients vs controls.
Also, in two studies where the SF-36 questionnaire was used for the assessment of the general quality of life and the OHIP-14 for oral quality of life, it was observed that the patients with the best level of oral problems had lower scores in the SF-36, indicating that oral quality of life had an effect on their overall quality of life.25,26 We also reported this correlation but using the SF-36 and the XeQoL questionnaires this time.
Regarding the measurement of the UWSF and its correlation with oral quality of life, in their study Rusthen et al., only found one weak correlation with the scoring of the OHIP-1422 questionnaire. In this study, we report a correlation of the UWSF and oral quality of life assessed by the XeQoL. We also identified lower saliva flow, a higher score in the EPSSRI index and a higher score in the VAS, the tool used to assess the symptoms which induce dry mouth over function, amount of saliva, and level of thirst in SS.16 Thus our patients mentioned symptoms regarding function, amount of saliva and degree of thirst. For example three quarters of the patients presented with dryness in the lips, and half of them with discomfort on swallowing, and a dry mouth and throat.
Finally we reported a correlation between the EPSSRI and oral quality of life assessed by the XeQoL, and also a correlation with the VAS, a finding which no study had previously explored.
Our study has limits in that the control group was recruited through a dental service serving the general population, where the majority of people who attended it did so for a specific dental problem, which would impact their oral quality of life or suggest greater prevalence of xerostomy. However, even so, we observed a poorer quality of life in the group of patients with PSS.
ConclusionThe results of our study enabled us to corroborate that patients with PSS have a poorer quality of life both in general terms and orally, in comparison with healthy individuals of the same age. Saliva flow is a factor which contributes to oral quality of life and the oral quality of life impacts on the general quality of life of the patients. Saliva flow in turn correlates with the appearance of oral symptoms associated with a dry mouth. The oral quality of life in patients with PSS could be improved with multidisciplinary healthcare by dentists and rheumatologists, with opportune intervention of symptomatic xerostomy therapy and the prevention of infections, tooth decay and the loss of teeth.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Fernández-Martínez G, Zamora-Legoff V, Hernández Molina G. Calidad de vida oral en pacientes con síndrome de Sjögren primario. Reumatol Clin. 2020;16:92–96.