Infections, including opportunistic infections, are a major and frequent cause of morbidity and mortality in patients with systemic autoimmune and rheumatic diseases. Pneumocystis jirovecii pneumonia, classically considered to be typical of HIV patients, transplanted patients or patients treated with oncological chemotherapy, is appearing increasingly frequently in these patients. Therefore, rheumatologists should know its mechanism of production, clinical manifestations, treatment and prophylaxis, all of which are addressed in this review.
Las infecciones, entre ellas las oportunistas, constituyen una causa importante y frecuente de morbilidad y mortalidad en los pacientes con enfermedades reumáticas y autoinmunes sistémicas. La neumonía por Pneumocystis jirovecii, clásicamente considerada propia de pacientes VIH, trasplantados o tratados con quimioterapia oncológica, aparece cada vez con mayor frecuencia en estos pacientes. Es por ello conveniente que los reumatólogos conozcan su mecanismo de producción, manifestaciones clínicas, tratamiento y profilaxis, aspectos todos ellos abordados en esta revisión.
Infections are a major and common cause of morbimortality in patients with rheumatic and systemic autoimmune diseases (RD&SA).1 They include opportunistic infections among others, defined as those caused by non-pathogenic organisms which become pathogenic when the immune system is altered.2 They are an increasingly severe problem, due to the use of immunosuppressor agents to treat diseases, as well as the increased susceptibility to infection caused by diseases themselves and the comorbidities associated with them.3,4
Pneumocystis jirovecii, which was previously known as Pneumocystis carinii, is a pathogenic, opportunistic and extracellular fungus that belongs to the hemiascomicetes class. It is in the Pneumocystis genus, a complex group that is composed of many species with peculiar characteristics that differentiate them from other fungi.5 Their cytoplasmatic membrane is mainly composed of cholesterol, unlike those of other fungi which have membranes that contain ergosterol. This is why standard antifungal drugs (such as the azoles and polyenes), are ineffective against P. jirovecii, as they act on the ergosterol of the fungal membrane, either directly (amphotericin) or indirectly, inhibiting its synthesis (azoles).6 The new antifungal drugs (echinocandins), nevertheless, interfere in the synthesis of 1,3-β-D glucan, a specific constituent of the membrane of all fungi that is found in high quantities in the cystic form of P. jirovecii, so that they would be a highly interesting member of the therapeutic arsenal.7 Another peculiarity of Pneumocystis that is unique in mycology is their specificity for the selective invasion of a concrete host, so that each mammal is affected by a different genetic variety. Only P. jirovecii is found in humans. Lastly, this fungus does not grow in artificial media, so that diagnosis requires demonstration that it is present in sputum samples obtained by broncoalveolar washing, or in tissue obtained by pulmonary biopsy, using microscopy techniques (preferentially with immunofluorescence), or by techniques involving highly sensitive polymerase chain reactions.8
Although its exact transmission mechanism is unknown, the most widely accepted hypothesis is that it passes from one person to another by the inhalation of particles in the air.8 Exposure to P. jirovecii in humans is highly common, as is shown by the fact that in developed countries more than 80% of children have developed antibodies against P. jirovecii before they are four years old.9
Although the theory used to be that pneumonia caused by P. jirovecii (NPJ) is due to the reactivation of a latent infection in an immunocompromised patient, current data show that it is a de novo infection. Nevertheless, colonisation by P. jirovecii has been shown to exist in certain susceptible populations, although the clinical implications of this finding are unclear.5
Once it has been acquired, P. jirovecii passes through a complicated life-cycle. It has two predominant forms, trophic and cystic, of which the first type represents 90% of the total number of P. jirovecii organisms during infection. Trophic forms are united by interdigitations to the cellular membrane of the type I pneumocyte in the alveolar epithelium, permitting the close unification of both membranes without breaking the alveolar cell or penetrating it. The interaction of P. jirovecii with the pneumocyte and alveolar macrophages triggers a cascade of cellular responses in P. jirovecii itself as well as in the lung cells: P. jirovecii is stimulated to proliferate, at the same time that the alveolar macrophages commence phagocytosis of P. jirovecii and its destruction, and the alveolar cells release proinflammatory cytokines and chemokines that promote the recruitment and activation of neutrophils and T lymphocytes. T CD4+ lymphocytes encharged with coordinating the inflammatory response of the host, recruiting and activating additional immune effecter cells (monocytes and macrophages) which will be responsible for eliminating P. jirovecii. In immunocompetent individuals the infection is eliminated with minimum inflammation and lung damage. However, in immunocompromised hosts a hyperinflammatory response is trigged that is unable to eliminate P. jirovecii but which causes lung damage and affects gas interchange. This causes NPJ, the chief manifestation of this infection.
Clinical manifestationsP. jirovecii has a special tropism for the lungs, and its dissemination in the rest of an infected organism is exceptional. Interstitial pneumonia is the main disease caused by P. jirovecii. The most common symptoms are the appearance of a dry cough, dyspnoea, fever, tachycardia and tachypnea.10 In pulmonary auscultation the presence of fine rales stands out, and in thoracic X-ray there is tenuous bilateral interstitial infiltrate. High resolution computed tomography (HRCT) shows higher sensitivity in detecting NPJ than simple X-ray imaging, showing the characteristic areas of ground glass opacity in peri-hiliar distribution.11 Generally high levels of lactate dehydrogenase (LDH) are detected, with a fall in serum albumin and CD4+ counts <200/mm3. Due to damage in the alveolar epithelium the blood gases are altered, detecting hypoxemia and/or respiratory failure in arterial gasometry.
In immunocompromised patients without HIV, the infection usually runs a more acute course, manifesting with fewer systemic symptoms but with greater respiratory repercussion (a higher level of respiratory failure and tachypnea), and in broncoalveolar washing a lower concentration of organisms is detected, although with a higher neutrophil count5 and, in general, the condition tends to be more severe, with a longer stay in intensive care units and higher rates of mortality.8,12 This higher mortality has been associated with low PaO2/FiO2 coefficients,13 with the need for mechanical ventilation,14 hypoalbuminemia, male sex, advanced age and medical care in private hospitals.15
The magnitude of the problemThis infection is often lethal.5 The mortality rate is close to 100% without treatment, and it varies from 5% to 40% in treated patients.10 The rate of mortality due to NPJ in patients without HIV currently stands at from 39.4% to 59.1%, a figure far higher than the 6%–7% recorded in HIV.14
The individuals at the greatest risk of NPJ are patients with HIV, especially those with CD4+ counts <200/mm;3 those who have received organ transplants or haematopoietic cells;5 premature infants who require mechanical ventilation; patients with primary immunodeficiencies that affect T lymphocyte functioning16 and patients who receive oncological chemotherapy or are treated with immunosuppressor drugs.17 The use of antiretroviral drugs to treat HIV infection together with prophylaxis against P. jirovecii following the recommendations of clinical practice guides18 have drastically reduced the incidence of NPJ in developed countries in patients with AIDS, from rates of 70%–80% before implementing these measures, to fewer than one case per 100 persons-year. On the contrary, an increase in incidence has been observed in immunocompromised patients without HIV: rates of incidence vary depending on the disease, but they may reach 44.6 cases per 100,000 patients/year in those who have received transplants, >45 cases per 100,000 patients/year in haematological neoplasias, 53.6 cases per 100,000 patients/year in inflammatory myopathies, 71.9 cases per100,000 patients/year in vasculitis associated with anti-neutrophil cytoplasm antibodies (ANCA) and 93.2 cases per 100,000 patients/year in patients with panarteritis nodosa (PAN).19
Guides for the prophylaxis of NPJ have recently been published for patients with haematological diseases and organ transplant.12 Nevertheless, to date no guide for the prophylaxis of NPJ has been published for patients with RD&SA under immunosuppressor treatment. Several authors have expressed the advisability of having these guides,20,21 as they would help to unify criteria which are currently very varied.22,23
As occurs in other pathologies,12 the risk of NPJ in patients with RD&SA will be determined by the combination of different factors: 1) the disease; 2) the drugs used; and 3) the particular circumstances of each individual. When deciding on the advisability of administering prophylaxis against P. jirovecii, it is also necessary to consider the potential toxicity of the treatment used for this.24 It has been suggested that a "level of risk of NPJ" higher than 3.5% should be the cut-off point for considering that the beneficial effect of prophylaxis is greater than the risk.25 Finally, it has to be taken into account that many of these factors will be modified over time, so that continuous re-evaluation will be required of the risk/benefit during the follow-up of the patients.26
Risks associated with the diseaseThe incidence of NPJ varies notably from some RD&SA to others, and it is higher in granulomatous vasculitis with polyangiitis (VGP), PAN, systemic lupus erythematosus (SLE) and inflammatory myopathies, making an individualised approach necessary.
ANCA-associated vasculitisANCA-associated vasculitis patients have a higher rate of NPJ incidence within RD&SA (8.9 cases per every 1,000 hospitalisations/year in VGP, 120/10.000 patients/years),15,27,28 with a very high mortality rate (47%–62.5%).1,15 The main factor associated with the development of NPJ in patients with VGP is lymphopenia: lymphocyte levels under 800/mm3 prior to treatment, or below 600/mm3 three months after the start of treatment, were associated with the development of NPJ in a retrospective study.29 Due to the high incidence of NPJ in these patients during induction treatment, the European guide for the management of ANCA-associated vasculitis recommends prophylaxis with TMP-SMX in all of the patients treated with cyclophosphamide (CFM), and it mentions its usefulness in maintaining remission as it reduces the risk of relapse.30 Other authors recommend prophylaxis during induction even when other drugs are given, such as rituximab, on condition that the dose of corticoid is higher than 10mg, as well as with lymphocyte counts below 300/mm.31
Other types of vasculitisThe incidence of NPJ in PAN is 6.5 cases per 1,000 hospitalisations/year,15 with a mortality of 47.6%.15
In large vessel arteritis incidence is surprisingly low,32 in spite of the use of high doses of corticoids over prolonged periods of time. None of the European management recommendations mentions NPJ prophylaxis.33–35
Systemic lupus erythematosus (SLE)There is no uniform criterion for indicating NPJ prophylaxis in patients with SLE: the overall incidence of this opportunist infection is lower than the level found in vasculitis-ANCA and inflammatory myopathies (5%,1 1.2 cases per 1,000 hospitalisations/year,15 8 cases per 10,000 patients/year28), although rates of mortality are high (20%–45.7%),15,36 which more than justifies its treatment. Numerous publications agree on the factors that predispose to the development of NPJ: disease that is more active, nephritis,36 the use of glucocorticoids (GC) at high doses, treatment with cyclophosphamide23 and, most especially, lymphopenia.27,29,36 Taking the recommendations for prophylaxis in patients with HIV as a guide,18 Lertnawapan et al.36 recommend starting prophylaxis when total levels of lymphocytes fall below 750/mm3 or when CD4+ <200/mm3.
There is special concern regarding the use of trimethoprim-sulfamethoxazole (TMP-SMX) in patients with SLE: high rates of adverse reactions have been reported in from 27.3%–53% of cases37 (cutaneous rash, exacerbation of the SLE, hepatic toxicity and myelosuppression),which is more frequent in patients who are anti-Ro/SS-A positive,38 although they are generally mild and do not require discontinuation of the drug.23,39 Nevertheless, this factor should be taken into account when recommending prophylaxis against P. jirovecii. It would therefore be desirable to have guides, recommendations or algorithms26 that would help clinicians to reach decisions on an individualised basis: unfortunately, the European guides for the management of SLE do not include any recommendations on this subject.40
Inflammatory myopathiesPatients with inflammatory myopathies have NPJ rates lower than those recorded for ANCA-associated vasculitis, although higher than in SLE, with figures that vary depending on the series from 2% to 10% of pacientes,1,41 and it is the cause in 27 cases of every 10,000 hospitalisations/year. As occurred in the previous cases, the rate of mortality is very high, at from 33% to 57.7%.15 Lymphopenia is once again the predisposing factor, so that some authors recommend starting prophylaxis when CD4+ lymphocyte counts are below 250/mm.41
Rheumatoid arthritisPatients with rheumatoid arthritis (RA) are generally considered to be low risk for the development of NPJ.15 Although the use of immunosuppressor drugs and biological agents increases the risk, the figures are below 0.1%-0.3%, with mortality rates that vary from 10% to 28.6%.42 There is also the additional risk of the MTX/TMP-SMX combination. Due to this, and although systematic prophylaxis is not recommended, some authors consider that certain preventive measures should be adopted, such as the detection of P. jirovecii carriers to implement treatment to eradicate it, thereby avoiding any subsequent prophylaxis.43
Drug associated riskGlucocorticoidsGC are an essential part of the arsenal for the treatment of a large number of RD&SA. The association between GC and the development of NPJ has been clearly established, considering it to be the main risk factor.17 Several mechanisms interconnect to facilitate infection: the prolonged use of GC causes a fall in T CD4+ lymphocytes, in the blood as well as in the lungs44 and, although it has not been clearly determined, it probably affects the functioning of alveolar macrophages, hindering phagocytosis and the destruction of P. jirovecii.
The risk of NPJ is dose and time-dependent, and doses higher than 60mg/day of prednisone lead to higher risk,24 although daily doses of 16mg for periods of eight weeks may be sufficient to induce NPJ.17 Nevertheless, it seems that another factor is necessary, given that in ACG the risk is low in spite of high doses of GC over a prolonged period of time.32 The type of disease, concomitant use of cyclophosphamide and the presence of lymphopenia have all been shown to be additional predisposing factors for the development of NPJ.24 Prophylaxis with TMP-SMX is highly effective,45 although in some studies this protective effect was only demonstrated when high doses of corticoids were used.24
There are not enough data in the literature to permit giving specific figures for dosage or treatment times over which prophylaxis should begin. A dose of prednisone of 16–20mg/day or higher has been suggested,17,46 as well as duration of treatment lasting eight weeks, after which prophylaxis is indicated.
In spite of all these data, curiously the EULRA recommendations on the use of GC do not mention either the risk or the advisability of prevention.47–49
Immunosuppressor drugsAlthough CFM has been implicated in the development of NPJ, in a dose-dependent relationship,29 its effect may be associated with other immunosuppressor factors such as the use of GC. Systematic prophylaxis is therefore not recommended, and treatment has to be individualised depending on the presence of other predisposing factors.
The role of other immunosuppressor drugs (methotrexate, azathioprine and mycophenolate) as predisposing factors for NPJ has not been clearly demonstrated.50
Biological agentsThere are currently no specific recommendations. The British Society of Rheumatology registry of biological treatments found a higher incidence in patients treated with rituximab respecting those treated with drugs that inhibit tumour necrosis factor (anti-TNF) (HR=3.2; CI 95%: 1.4–7.5).51 Given the low incidence in the majority of the series (0.03%-0.3%)42 systematic prophylaxis is not recommended for all patients receiving biological treatment, although it would be of interest to identify the subgroups at especial risk which could benefit from this prophylaxis.52
Risk associated with individual factorsLymphopeniaLymphopenia has been shown to be one of the main predisposing factors for NPJ in patients with RD&SA.13,15,27,29,41,50,53 The clinical practice guides for patients with HIV, indicate starting prophylaxis against P. jirovecii when levels of CD4+ are below 200/mm.318 Several studies find counts of below 250/mm3 in all of the patients who develop NPJ.27,54 Although the evidence is not so solid, in patients with RD&SA several authors recommend starting prophylaxis based on the same criterion.55
Total lymphocyte levels prior to starting treatment have also be shown to be useful as a predictive factor: in VGP, NPJ is associated with pre-treatment lymphocyte levels below 800/mm3;29 Porges et al.56 find figures under 350/mm3 identify all of the patients with SLE treated with GC and cytotoxic drugs in the risk of NPJ; and in a heterogeneous group of connective tissue pathologies Ogawa et al.53 found an association between counts below 500/mm3 two weeks before starting treatment with corticoids at doses higher than 30mg/day. Although it seems that absolute lymphopenia really is a predisposing factor for NPJ, nobody has dared to set a cut-off point under which prophylaxis is indicated.
The drug of choice for prophylaxisDifferent drugs have been tested in the prophylaxis of NPJ: TMP/SMX, pentamidine, atovaquone, dapsone, pyrimethamine and clindamycin.57 The best result was obtained with TMP/SMX, and this is why it is the recommended first-line drug. Daily administration is equally effective as administration three days per week.21,58 When TMP-SMX is contraindicated the recommended second-line is pentamidine, atovaquone or dapsone20 (Table 1).
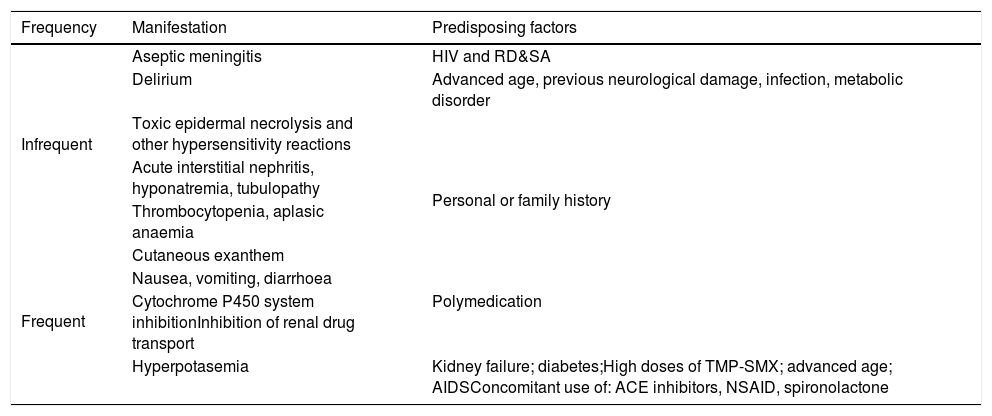
It is important to take into account the interaction between TMP-SMX and methotrexate, (MTX) as this association may cause severe cytopenia and myelosuppression, even at low doses of MTX over a short time (two days) of treatment with TMP-SMX.59 This effect does not seem to arise with prophylactic doses of TMP-SMX, although it must be taken into account. The main side effects associated with the use of TMP/SMX are listed in Table 2.60,61
Side effects of TMP-SMX.
| Frequency | Manifestation | Predisposing factors |
|---|---|---|
| Infrequent | Aseptic meningitis | HIV and RD&SA |
| Delirium | Advanced age, previous neurological damage, infection, metabolic disorder | |
| Toxic epidermal necrolysis and other hypersensitivity reactions | Personal or family history | |
| Acute interstitial nephritis, hyponatremia, tubulopathy | ||
| Thrombocytopenia, aplasic anaemia | ||
| Frequent | Cutaneous exanthem | |
| Nausea, vomiting, diarrhoea | ||
| Cytochrome P450 system inhibitionInhibition of renal drug transport | Polymedication | |
| Hyperpotasemia | Kidney failure; diabetes;High doses of TMP-SMX; advanced age; AIDSConcomitant use of: ACE inhibitors, NSAID, spironolactone |
The duration of prophylaxis is controversial. In ANCA-associated vasculitis it is suggested that it be discontinued when the immunosuppressant is suspended, when the corticoid dose is lower than 20−10mg of prednisone/day, or when B lymphocyte depletion halts after having used RTX.21 Prior to discontinuation other factors should be evaluated: lymphopenia, leucopoenia, the CD4+ count and the dose of corticoid.
ConclusionsThere can be no doubt that the incidence of NPJ is higher in patients with certain RD&SA, often with a disastrous outcome. Nor can there be any doubt as to the efficacy of prophylaxis in preventing it. However, there is no clear definition of the circumstances under which it is reasonable to apply the said prophylaxis. This uncertainty is reflected in the result of different surveys of clinics, where a wide range of criteria were detected.22,23
Green et al.25 set the cut-off point at a risk of NPJ above 3.5% so that the risk/benefit balance is favourable. According to these authors, patients with VGP would be included in this category, while the other RD&SA (inflammatory myopathies, SLE, PAN, scleroderma and RA) should not receive prophylaxis as the balance would be unfavourable. In their study Park et al.24 find an overall number necessary for harm (NNH) of 131 (55–∞), as opposed to a number necessary to prevent a case of NPJ (NNT) of 52 (33–124). Stratifying according to diseases, the NNT in patients with SLE (43 (28–85)) or PAM (3 (1.6–39.4)) was lower than the NNH, which would justify prophylaxis. This is not the case for the other diseases. Nevertheless, the actual situation is far more complex, as the risk of NPJ will not only depend on the type of disease, but also on its association with other predisposing factors. A retrospective review of 21 cases of NPJ in patients with RD&SA over a period of 20 years in an American hospital supports the theory that aetiology here is multifactorial, with the participation of several factors (high doses of corticoids, the use of other immunosuppressor drugs, lymphopenia or the coexistence of interstitial pulmonary disease).62 None of the 21 patients had received prophylaxis.
Different authors propose a very wide range of types of prophylaxis:
- •
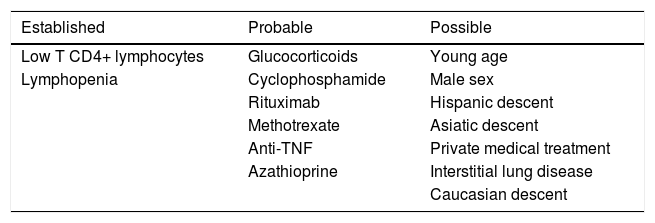
Wolfe et al.21 classify NPJ risk factors into three categories: established, probable and possible (Table 3). They suggest commencing prophylaxis:
- ◦
Always, during induction treatment in VGP.
- ◦
In other ANCA-associated vasculitis and in PAN: during induction treatment or high dose corticoids, on condition that there is lymphopenia (<500cells/mm3) or a low CD4+ count (<200/mm3).
- ◦
In SLE and inflammatory myopathies: when high doses of corticoids are being administered, when there is also lymphopenia or a low CD4+ count and in SLE, during immunosuppressor treatment or severe disease in case of inflammatory myopathy.
Table 3.Risk factors for the development of NPJ in patients with RD&SA. Modified from Wolf et al.21.
Established Probable Possible Low T CD4+ lymphocytes Glucocorticoids Young age Lymphopenia Cyclophosphamide Male sex Rituximab Hispanic descent Methotrexate Asiatic descent Anti-TNF Private medical treatment Azathioprine Interstitial lung disease Caucasian descent - ◦
- •
Ogawa et al.,53 Chew et al.63 and Yale et al.17 base the start of prophylaxis on the corticoid dose given, and they propose commencing it when the dose is above 10−30mg of prednisone.
- •
Stamp et al.20 administer prophylaxis to all of their patients treated with immunosuppressants and those with a history of NPJ, as well as patients with persistent lymphopenia and CD4+ counts below 200/mm3.
- •
Demoruelle et al.64 propose reserving prophylaxis for patients with connective tissue pathologies who fulfil two or more of the following criteria:
- ◦
Steroids ≥20mg/day for more than four weeks.
- ◦
≥ Two disease-modifying drugs (DMD).
- ◦
Total lymphocyte count ≤ 350/mm3.
- ◦
Parenchymal lung disease.
- ◦
- •
Gupta et al.23 propose, in patients with SLE treated with CFM, administering prophylaxis only to those who have an additional risk factor: severe leucopoenia, severe lymphopenia, high doses of corticoids, hypocomplementemia, active kidney disease or high activity scores (SLEDAI).
- •
Li et al.27 propose administering prophylaxis to patients with RD&SA who receive high doses of immunosuppressant (such as pulses of methyl-prednisolone) and who have CD4+ counts lower than 250/mm3.
- •
Sowden et al.55 propose determining CD4+ after one month of immunosuppressant only in patients who fulfil the following three criteria: doses of prednisolone or equivalent above 15mg/day, planned duration of treatment longer than three months, and total lymphocyte counts <600/mm3. In cases with CD4+ counts <200, prophylaxis would commence if the annual risk of P. jirovecii is higher than 9%.
- •
Mansharamani et al.,54 suggest use a CD4+ count lower than 300/mm3 as the cut-off point to commence prophylaxis.
- •
Mori and Sugimoto43 administer one tablet of TMP-SMX (80mg TMX plus 400mg SMX) per day during 5–7 days, or two tablets three days a week for one week, to all patients with RA prior to starting treatment.
At the current time there are no data on which to decide which of these guidelines is the most effective in clinical practice, and nor have any studies been designed to find predictive factors for the development of NPJ in patients with RD&SA.65 Taking its severity and the possibility of preventing it into account, and awaiting studies that supply more solid and conclusive data, it would be desirable to have recommendations that help clinicians to identify risk situations and apply prophylaxis more efficiently, as this would doubtless have a highly favourable repercussion in our patients.
FinancieringThis research received no specific aid from public, private or not-for-profit bodies.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Vela Casasempere P, Ruiz Torregrosa P, García Sevila R. Pneumocystis jirovecii en pacientes inmunocomprometidos con enfermedades reumáticas. Reumatol Clin. 2021;17:290–296.