The coronavirus disease 2019 (COVID-19) is the result of infection with the SARS-CoV-2 virus that is making us live one of the worst pandemics of the 21st century. It has affected the management of rheumatoid arthritis (RA), since these patients are treated with immunosuppressants such as disease-modifying drugs and biologics (bDMARDs). At the beginning of the pandemic, when a patient with RA required hospital admission for COVID-19, immunosuppression was suspended until improvement.1 Over time, we have learned about SARS-CoV-2 infection, showing that use of bDMARDs is not associated with worse outcomes2 except for rituximab and JAK inhibitors.3
However, the management is challenging. When a patient with RA without prior interstitial lung disease (ILD) is hospitalized with severe SARS-CoV-2 pneumonia passes the acute phase, and suffers a flare of RA, there is an understandable concern to reintroduce immunosuppression. In this phase, the patient is normally still on low-dose steroid treatment, but the bDMARDs that can be used for SARS-CoV-2 infection have been stopped (such as tocilizumab or bariticinib, also useful for RA). Therefore, the rheumatologist must decide to reintroduce the RA baseline treatment or to change it due to the new ILD secondary to the virus. We would like to share our experience.
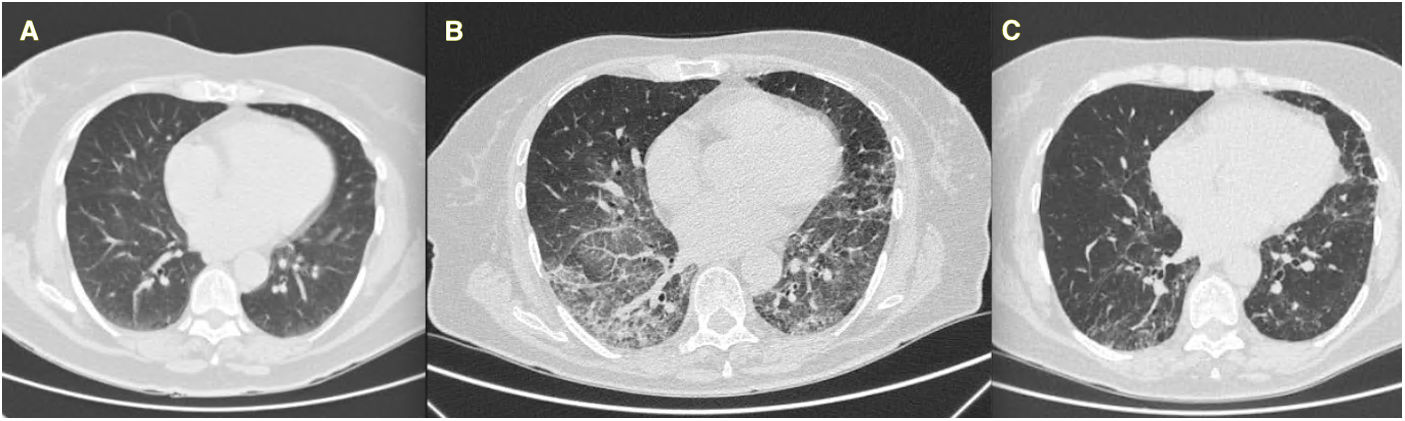
We present the case of a 72-year-old woman with RA since 1997 treated with 25mg of methotrexate (MTX) weekly and 125mg of abatacept weekly, in remission. In 2018 she complained of a dry cough and was studied with CT scan (normal, Fig. 1A), and finally diagnosed with gastric reflux.
In March 2020, she was admitted to the intensive care unit for severe global bilateral SARS-CoV-2 pneumonia. MTX and abatacept were suspended, she was intubated and treated with corticosteroids, antivirals, and later tocilizumab. Her evolution was favorable, but on the 40th day of hospitalization, despite maintaining 0.5mg/kg of prednisone, she presented a severe polyarticular RA flare. At that time, MTX and abatacept were reintroduced. She has been in remission to date.
In September 2020, one month after hospital discharge, she was still reporting stable dyspnea on moderate efforts, the CT scan was as shown in Fig. 1B (ground glass areas and thickening of interlobular septa in both lower lobes, patchy ground consolidation in all lobes, and honeycomb changes in the left upper lobe), and the functional tests were as follows: FEV1 2140ml (104% of predicted value), FVC 2620ml (99%), DLco (corrected for hemoglobin) 3030mmol/kPa/min (49%). She was treated by pneumologists with a descending dose of 0.5mg/kg of prednisone for a month. Six months later functional tests improved – FEV1 2240ml (111%), FVC 2740ml (105%), DLco 3950mmol/kPa/min (64%) – as well as the CT scan (Fig. 1C). To date, she has not reported dyspnea and her baseline saturation is 99%. She has maintained MTX and abatacept without any adverse event or RA flare. NSAIDs or other corticosteroids have not been needed.
There is another report of a RA patient maintaining 10mg of MTX during the hospitalization period, and her condition remained stable.4 MTX does not have an increased risk of infections 5and is not associated with an increased risk of RA-ILD in RA.6 We suggest, sustained on current published data,7–9 that reintroducing baseline treatment when RA patients surpass the critic phase of COVID-19 disease does not worsen the evolution of ILD secondary to SARS-CoV-2, as it appears to be independent. However, given that the bDMARD in our case is abatacept, which has a good ILD profile in patients with RA, it would be of great interest to collect more data with all DMARDs and bDMARDs in these patients.