Pregnancy and puerperium are considered a risk situation in women with systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS). Therefore, specialised assessment is essential both preconception and during pregnancy and the puerperium. Likewise, it is very important that different specialists in autoimmune diseases and high-risk pregnancies participate in the follow-up of these patients in a coordinated manner. The Spanish Society of Gynaecology and Obstetrics, the Spanish Society of Internal Medicine, and the Spanish Society of Rheumatology have set up a working group for the preparation of three consensus documents.
MethodsThe stages of the work were: distribution of work in three groups corresponding to the three periods related to pregnancy (preconception, during pregnancy and childbirth and puerperium), identification of key areas, exhaustive review of the literature and formulation of recommendations.
Results161 recommendations were prepared that address the three periods indicated. This first document includes the 48 recommendations that address aspects related to infertility, the need for and treatments for gonadal preservation and preconception assessment.
ConclusionsThese multidisciplinary recommendations are considered decision-making tools for clinicians involved in the care of patients with SLE/APS during pregnancy.
El embarazo y el puerperio se consideran una situación de riesgo en mujeres con lupus eritematoso sistémico (LES) y síndrome antifosfolípido (SAF). Es esencial que especialistas en enfermedades autoinmunes y en embarazo de alto riesgo intervengan en su seguimiento de forma coordinada. La Sociedad Española de Ginecología y Obstetricia, la Sociedad Española de Medicina Interna, y la Sociedad Española de Reumatología han constituido un grupo de trabajo paritario para la elaboración de 3 documentos de consenso.
MétodosLas fases del trabajo fueron: distribución del trabajo en grupos correspondientes a los 3 períodos relacionados con la gestación, identificación de áreas clave, revisión de la literatura y formulación de recomendaciones.
ResultadosEn este primer documento se incluyen las primeras 48 recomendaciones que tratan aspectos relacionados con la infertilidad, la necesidad y los tratamientos de preservación gonadal y la valoración preconcepcional.
ConclusionesEstas recomendaciones multidisciplinares se consideran herramientas en la toma de decisiones para los clínicos involucrados en la asistencia a pacientes con LES/SAF durante el embarazo.
Pregnancy and the postpartum are considered risk situations for women with autoimmune diseases. With respect to systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS) there is an increased risk of both maternal complications in the form of flare-ups of disease activity and thrombosis, and foetal complications in the form of early and late losses, intrauterine growth restriction and hypertensive disorders of pregnancy. Therefore, assessment is essential first of all, preconception and during pregnancy and the puerperium. Secondly, it is very important that different specialists in autoimmune diseases and high-risk pregnancy are involved in the follow-up of these patients in a coordinated manner.
In recent years, several scientific societies have issued recommendations on pregnancy control in patients with SLE/ASP.1,2 However, some points remain controversial and others have not been addressed. In order to reach a consensus on some of these aspects related to the preconception period, and treatment and follow-up during pregnancy and the postpartum in patients with SLE/APS, the Spanish Society of Gynaecology and Obstetrics (SEGO), the Systemic Autoimmune Diseases Group (GEAS) of the Spanish Society of Internal Medicine (SEMI), and the Systemic Autoimmune Diseases Group (EASSER) of the Spanish Society of Rheumatology (SER) formed a joint working group to draw up 3 consensus documents after a critical review of the literature.
In this first document, aspects are discussed related to infertility, the need for and treatments for gonadal preservation and preconception assessment in women with SLE/APS.
Material and methodsThe recommendations were developed following the Delphi methodology.3 Each of the scientific societies involved proposed 5 representatives from each based on their experience in the area of pregnancy control in patients with autoimmune diseases. After establishing a nominal group, work was distributed into 3 groups corresponding to the 3 periods related to gestation: preconception, during pregnancy and delivery, and postpartum. Each group was made up of a specialist in Gynaecology and Obstetrics, one in Internal Medicine and another in Rheumatology (except the pregnancy group, which had 2 representatives from each specialty), plus a coordinator for each of the societies. Each group performed an exhaustive review of the literature according to the questions posed in each section.
Nominal group meetingsA total of 3 face-to-face meetings were held. In the first meeting different strategies for drawing up this document were discussed. After a vote, the panel opted to design the document in the form of questions that would preferentially consider aspects not included in the recent recommendations.1,2 In the other 2 meetings, the draft of each section was worked on and the levels of evidence and degrees of recommendation were reviewed.
Literature reviewA systematic review of the published literature was undertaken,4,5 which was broadened with a narrative review, including the bibliographic references described in the articles reviewed, relating to the different questions of the consensus. Medline was used as the bibliographic database (from inception to November 2016). Finally, the following types of studies were included: meta-analyses, systematic and non-systematic reviews, randomised and non-randomised clinical trials but including a control group, and case–control studies.
The search criteria for SLE were:
(“lupus erythematosus, systemic/complications”[Mesh Terms] OR “lupus erythematosus, systemic/congenital”[Mesh Terms] OR “lupus erythematosus, systemic/drug therapy”[Mesh Terms] OR “lupus erythematosus, systemic/mortality”[Mesh Terms] OR ((“lupus erythematosus, systemic”[MeSH Terms] OR (“lupus”[All Fields] AND “erythematosus”[All Fields] AND “systemic”[All Fields]) OR “systemic lupus erythematosus”[All Fields] OR (“lupus”[All Fields] AND “erythematosus”[All Fields] AND “systemic”[All Fields]) OR “lupus erythematosus, systemic”[All Fields]) AND “pharmacology”[MeSH Terms]) OR “lupus erythematosus, systemic/prevention and control”[Mesh Terms] OR “lupus erythematosus, systemic/therapy”[Mesh Terms]) AND (“pregnancy”[MeSH Terms] OR (“pregnancy”[MeSH Terms] OR “pregnancy”[All Fields])) AND ((Clinical Trial[ptyp] OR Comparative Study[ptyp] OR Consensus Development Conference, NIH[ptyp] OR Controlled Clinical Trial[ptyp] OR Guideline[ptyp] OR Meta-Analysis[ptyp] OR Multicenter Study[ptyp] OR Observational Study[ptyp] OR Practice Guideline[ptyp] OR Randomised Controlled Trial[ptyp] OR Review[ptyp] OR systematic[sb]) AND (“2004/01/01”[PDAT]: “2016/10/30”[PDAT]) AND “humans”[MeSH Terms] AND (English[lang] OR Spanish[lang])).
The search criteria for APS were:
(“antiphospholipid syndrome/complications”[Mesh Terms] OR “antiphospholipid syndrome/congenital”[Mesh Terms] OR “antiphospholipid syndrome/drug therapy”[Mesh Terms] OR “antiphospholipid syndrome/embryology”[Mesh Terms] OR “antiphospholipid syndrome/genetics”[Mesh Terms] OR “antiphospholipid syndrome/mortality”[Mesh Terms] OR “antiphospholipid syndrome/prevention and control”[Mesh Terms] OR “antiphospholipid syndrome/therapy”[Mesh Terms]) AND (“pregnancy”[MeSH Terms] OR (“pregnancy”[MeSH Terms] OR “pregnancy”[All Fields])) AND ((Clinical Trial[ptyp] OR Comparative Study[ptyp] OR Consensus Development Conference, NIH[ptyp] OR Controlled Clinical Trial[ptyp] OR Guideline[ptyp] OR Meta-Analysis[ptyp] OR Multicenter Study[ptyp] OR Observational Study[ptyp] OR Practice Guideline[ptyp] OR Randomized Controlled Trial[ptyp] OR Review[ptyp] OR systematic[sb]) AND (“2004/01/01”[PDAT]: “2016/10/30”[PDAT]) AND “humans”[MeSH Terms] AND (English[lang] OR Spanish[lang])).
DelphiAfter the review and having answered the different questions with their degree of evidence, a series of recommendations were elaborated and submitted to an online Delphi vote. The Delphi vote was made by all members of the nominal group. Each member voted on each of the recommendations on a scale of 1–10 (where 1=totally disagree and 10=totally agree). Agreement was defined when at least 80% of the participants had given a value ≥7. Recommendations where the degree of agreement was less than 80% were re-evaluated and discussed, the vote being reassessed in a second round or the disagreement maintained when so warranted by the explanation (Table 1).
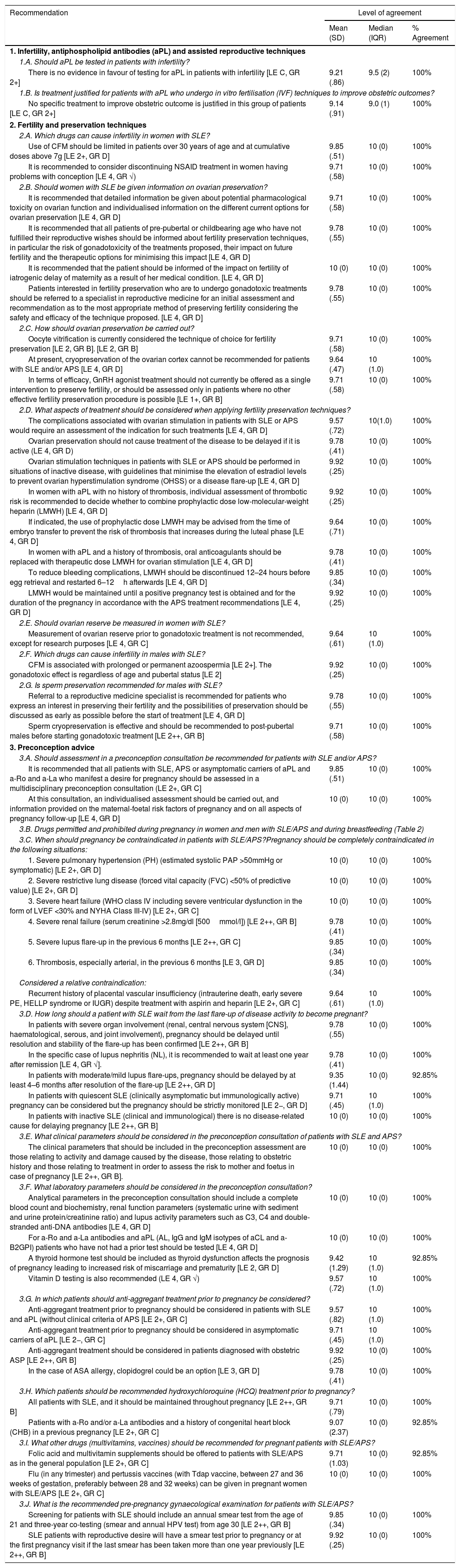
Recommendations on infertility, ovarian preservation and preconception assessment in patients with systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS).
| Recommendation | Level of agreement | ||
|---|---|---|---|
| Mean (SD) | Median (IQR) | % Agreement | |
| 1. Infertility, antiphospholipid antibodies (aPL) and assisted reproductive techniques | |||
| 1.A. Should aPL be tested in patients with infertility? | |||
| There is no evidence in favour of testing for aPL in patients with infertility [LE C, GR 2+] | 9.21 (.86) | 9.5 (2) | 100% |
| 1.B. Is treatment justified for patients with aPL who undergo in vitro fertilisation (IVF) techniques to improve obstetric outcomes? | |||
| No specific treatment to improve obstetric outcome is justified in this group of patients [LE C, GR 2+] | 9.14 (.91) | 9.0 (1) | 100% |
| 2. Fertility and preservation techniques | |||
| 2.A. Which drugs can cause infertility in women with SLE? | |||
| Use of CFM should be limited in patients over 30 years of age and at cumulative doses above 7g [LE 2+, GR D] | 9.85 (.51) | 10 (0) | 100% |
| It is recommended to consider discontinuing NSAID treatment in women having problems with conception [LE 4, GR √) | 9.71 (.58) | 10 (0) | 100% |
| 2.B. Should women with SLE be given information on ovarian preservation? | |||
| It is recommended that detailed information be given about potential pharmacological toxicity on ovarian function and individualised information on the different current options for ovarian preservation [LE 4, GR D] | 9.71 (.58) | 10 (0) | 100% |
| It is recommended that all patients of pre-pubertal or childbearing age who have not fulfilled their reproductive wishes should be informed about fertility preservation techniques, in particular the risk of gonadotoxicity of the treatments proposed, their impact on future fertility and the therapeutic options for minimising this impact [LE 4, GR D] | 9.78 (.55) | 10 (0) | 100% |
| It is recommended that the patient should be informed of the impact on fertility of iatrogenic delay of maternity as a result of her medical condition. [LE 4, GR D] | 10 (0) | 10 (0) | 100% |
| Patients interested in fertility preservation who are to undergo gonadotoxic treatments should be referred to a specialist in reproductive medicine for an initial assessment and recommendation as to the most appropriate method of preserving fertility considering the safety and efficacy of the technique proposed. [LE 4, GR D] | 9.78 (.55) | 10 (0) | 100% |
| 2.C. How should ovarian preservation be carried out? | |||
| Oocyte vitrification is currently considered the technique of choice for fertility preservation [LE 2, GR B]. [LE 2, GR B] | 9.71 (.58) | 10 (0) | 100% |
| At present, cryopreservation of the ovarian cortex cannot be recommended for patients with SLE and/or APS [LE 4, GR D] | 9.64 (.47) | 10 (1.0) | 100% |
| In terms of efficacy, GnRH agonist treatment should not currently be offered as a single intervention to preserve fertility, or should be assessed only in patients where no other effective fertility preservation procedure is possible [LE 1+, GR B] | 9.71 (.58) | 10 (0) | 100% |
| 2.D. What aspects of treatment should be considered when applying fertility preservation techniques? | |||
| The complications associated with ovarian stimulation in patients with SLE or APS would require an assessment of the indication for such treatments [LE 4, GR D] | 9.57 (.72) | 10(1.0) | 100% |
| Ovarian preservation should not cause treatment of the disease to be delayed if it is active (LE 4, GR D) | 9.78 (.41) | 10 (0) | 100% |
| Ovarian stimulation techniques in patients with SLE or APS should be performed in situations of inactive disease, with guidelines that minimise the elevation of estradiol levels to prevent ovarian hyperstimulation syndrome (OHSS) or a disease flare-up [LE 4, GR D] | 9.92 (.25) | 10 (0) | 100% |
| In women with aPL with no history of thrombosis, individual assessment of thrombotic risk is recommended to decide whether to combine prophylactic dose low-molecular-weight heparin (LMWH) [LE 4, GR D] | 9.92 (.25) | 10 (0) | 100% |
| If indicated, the use of prophylactic dose LMWH may be advised from the time of embryo transfer to prevent the risk of thrombosis that increases during the luteal phase [LE 4, GR D] | 9.64 (.71) | 10 (0) | 100% |
| In women with aPL and a history of thrombosis, oral anticoagulants should be replaced with therapeutic dose LMWH for ovarian stimulation [LE 4, GR D] | 9.78 (.41) | 10 (0) | 100% |
| To reduce bleeding complications, LMWH should be discontinued 12–24 hours before egg retrieval and restarted 6–12h afterwards [LE 4, GR D] | 9.85 (.34) | 10 (0) | 100% |
| LMWH would be maintained until a positive pregnancy test is obtained and for the duration of the pregnancy in accordance with the APS treatment recommendations [LE 4, GR D] | 9.92 (.25) | 10 (0) | 100% |
| 2.E. Should ovarian reserve be measured in women with SLE? | |||
| Measurement of ovarian reserve prior to gonadotoxic treatment is not recommended, except for research purposes [LE 4, GR C] | 9.64 (.61) | 10 (1.0) | 100% |
| 2.F. Which drugs can cause infertility in males with SLE? | |||
| CFM is associated with prolonged or permanent azoospermia [LE 2+]. The gonadotoxic effect is regardless of age and pubertal status [LE 2] | 9.92 (.25) | 10 (0) | 100% |
| 2.G. Is sperm preservation recommended for males with SLE? | |||
| Referral to a reproductive medicine specialist is recommended for patients who express an interest in preserving their fertility and the possibilities of preservation should be discussed as early as possible before the start of treatment [LE 4, GR D] | 9.78 (.55) | 10 (0) | 100% |
| Sperm cryopreservation is effective and should be recommended to post-pubertal males before starting gonadotoxic treatment [LE 2++, GR B] | 9.71 (.58) | 10 (0) | 100% |
| 3. Preconception advice | |||
| 3.A. Should assessment in a preconception consultation be recommended for patients with SLE and/or APS? | |||
| It is recommended that all patients with SLE, APS or asymptomatic carriers of aPL and a-Ro and a-La who manifest a desire for pregnancy should be assessed in a multidisciplinary preconception consultation (LE 2+, GR C] | 9.85 (.51) | 10 (0) | 100% |
| At this consultation, an individualised assessment should be carried out, and information provided on the maternal-foetal risk factors of pregnancy and on all aspects of pregnancy follow-up [LE 4, GR D] | 10 (0) | 10 (0) | 100% |
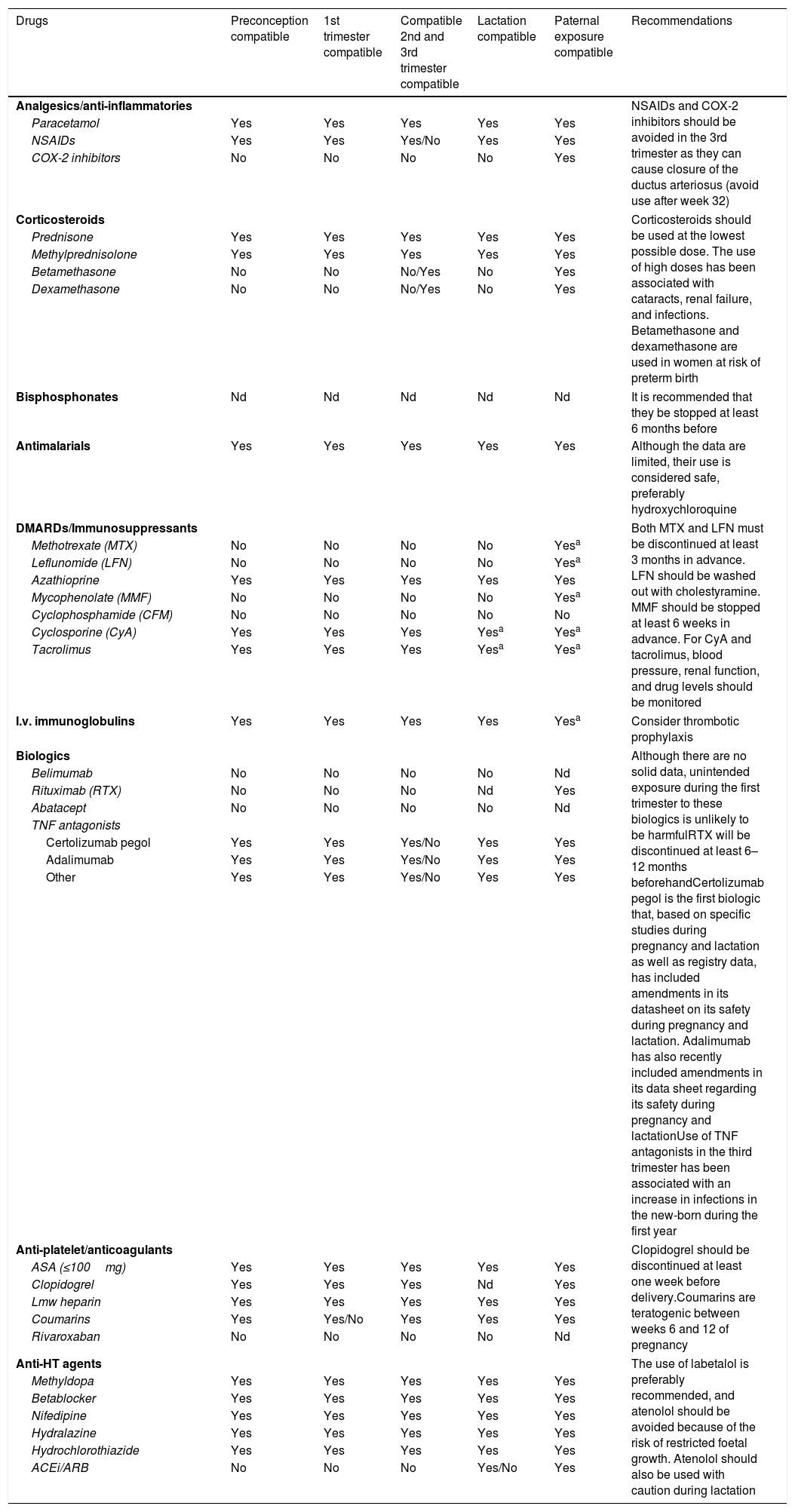
| 3.B. Drugs permitted and prohibited during pregnancy in women and men with SLE/APS and during breastfeeding (Table 2) | |||
| 3.C. When should pregnancy be contraindicated in patients with SLE/APS?Pregnancy should be completely contraindicated in the following situations: | |||
| 1. Severe pulmonary hypertension (PH) (estimated systolic PAP >50mmHg or symptomatic) [LE 2+, GR D] | 10 (0) | 10 (0) | 100% |
| 2. Severe restrictive lung disease (forced vital capacity (FVC) <50% of predictive value) [LE 2+, GR D] | 10 (0) | 10 (0) | 100% |
| 3. Severe heart failure (WHO class IV including severe ventricular dysfunction in the form of LVEF <30% and NYHA Class III-IV) [LE 2+, GR C] | 10 (0) | 10 (0) | 100% |
| 4. Severe renal failure (serum creatinine >2.8mg/dl [500mmol/l]) [LE 2++, GR B] | 9.78 (.41) | 10 (0) | 100% |
| 5. Severe lupus flare-up in the previous 6 months [LE 2++, GR C] | 9.85 (.34) | 10 (0) | 100% |
| 6. Thrombosis, especially arterial, in the previous 6 months [LE 3, GR D] | 9.85 (.34) | 10 (0) | 100% |
| Considered a relative contraindication: | |||
| Recurrent history of placental vascular insufficiency (intrauterine death, early severe PE, HELLP syndrome or IUGR) despite treatment with aspirin and heparin [LE 2+, GR C] | 9.64 (.61) | 10 (1.0) | 100% |
| 3.D. How long should a patient with SLE wait from the last flare-up of disease activity to become pregnant? | |||
| In patients with severe organ involvement (renal, central nervous system [CNS], haematological, serous, and joint involvement), pregnancy should be delayed until resolution and stability of the flare-up has been confirmed [LE 2++, GR B] | 9.78 (.55) | 10 (0) | 100% |
| In the specific case of lupus nephritis (NL), it is recommended to wait at least one year after remission [LE 4, GR √]. | 9.78 (.41) | 10 (0) | 100% |
| In patients with moderate/mild lupus flare-ups, pregnancy should be delayed by at least 4–6 months after resolution of the flare-up [LE 2++, GR D] | 9.35 (1.44) | 10 (0) | 92.85% |
| In patients with quiescent SLE (clinically asymptomatic but immunologically active) pregnancy can be considered but the pregnancy should be strictly monitored [LE 2−, GR D] | 9.71 (.45) | 10 (1.0) | 100% |
| In patients with inactive SLE (clinical and immunological) there is no disease-related cause for delaying pregnancy [LE 2++, GR B] | 10 (0) | 10 (0) | 100% |
| 3.E. What clinical parameters should be considered in the preconception consultation of patients with SLE and APS? | |||
| The clinical parameters that should be included in the preconception assessment are those relating to activity and damage caused by the disease, those relating to obstetric history and those relating to treatment in order to assess the risk to mother and foetus in case of pregnancy [LE 2++, GR B]. | 10 (0) | 10 (0) | 100% |
| 3.F. What laboratory parameters should be considered in the preconception consultation? | |||
| Analytical parameters in the preconception consultation should include a complete blood count and biochemistry, renal function parameters (systematic urine with sediment and urine protein/creatinine ratio) and lupus activity parameters such as C3, C4 and double-stranded anti-DNA antibodies [LE 4, GR D] | 10 (0) | 10 (0) | 100% |
| For a-Ro and a-La antibodies and aPL (AL, IgG and IgM isotypes of aCL and a-B2GPI) patients who have not had a prior test should be tested [LE 4, GR D] | 10 (0) | 10 (0) | 100% |
| A thyroid hormone test should be included as thyroid dysfunction affects the prognosis of pregnancy leading to increased risk of miscarriage and prematurity [LE 2, GR D] | 9.42 (1.29) | 10 (1.0) | 92.85% |
| Vitamin D testing is also recommended (LE 4, GR √) | 9.57 (.72) | 10 (1.0) | 100% |
| 3.G. In which patients should anti-aggregant treatment prior to pregnancy be considered? | |||
| Anti-aggregant treatment prior to pregnancy should be considered in patients with SLE and aPL (without clinical criteria of APS [LE 2+, GR C] | 9.57 (.82) | 10 (1.0) | 100% |
| Anti-aggregant treatment prior to pregnancy should be considered in asymptomatic carriers of aPL [LE 2−, GR C] | 9.71 (.45) | 10 (1.0) | 100% |
| Anti-aggregant treatment should be considered in patients diagnosed with obstetric ASP [LE 2++, GR B] | 9.92 (.25) | 10 (0) | 100% |
| In the case of ASA allergy, clopidogrel could be an option [LE 3, GR D] | 9.78 (.41) | 10 (0) | 100% |
| 3.H. Which patients should be recommended hydroxychloroquine (HCQ) treatment prior to pregnancy? | |||
| All patients with SLE, and it should be maintained throughout pregnancy [LE 2++, GR B] | 9.71 (.79) | 10 (0) | 100% |
| Patients with a-Ro and/or a-La antibodies and a history of congenital heart block (CHB) in a previous pregnancy [LE 2+, GR C] | 9.07 (2.37) | 10 (0) | 92.85% |
| 3.I. What other drugs (multivitamins, vaccines) should be recommended for pregnant patients with SLE/APS? | |||
| Folic acid and multivitamin supplements should be offered to patients with SLE/APS as in the general population [LE 2+, GR C] | 9.71 (1.03) | 10 (0) | 92.85% |
| Flu (in any trimester) and pertussis vaccines (with Tdap vaccine, between 27 and 36 weeks of gestation, preferably between 28 and 32 weeks) can be given in pregnant women with SLE/APS [LE 2+, GR C] | 10 (0) | 10 (0) | 100% |
| 3.J. What is the recommended pre-pregnancy gynaecological examination for patients with SLE/APS? | |||
| Screening for patients with SLE should include an annual smear test from the age of 21 and three-year co-testing (smear and annual HPV test) from age 30 [LE 2++, GR B] | 9.85 (.34) | 10 (0) | 100% |
| SLE patients with reproductive desire will have a smear test prior to pregnancy or at the first pregnancy visit if the last smear has been taken more than one year previously [LE 2++, GR B] | 9.92 (.25) | 10 (0) | 100% |
SD: standard deviation; GR: grade of recommendation; LE: level of evidence; IQR: interquartile range.
The final consensus document for each section was drafted with all the previous material. The document was circulated among the panel members for comments from each party. And finally, the final document was drafted with the final recommendations. Each of the recommendations was assigned a level of evidence (LE) and a grade of recommendation (GR) using the modified SIGN system based on the Oxford2 Centre for Evidence-Based Medicine (CMBE).6
Finally, the final document was sent to all group members for their final comments and corrections.
RecommendationsTable 1 shows the recommendations of Part 1 (infertility, ovarian preservation and preconception assessment) of this consensus document. Table 2 shows the effect on pregnancy and lactation of the drugs used in the treatment of SLE and ASP. The full version of the article can be accessed online.
Effect on pregnancy and lactation of drugs used in the treatment of systemic lupus erythematosus and antiphospholipid syndrome.
| Drugs | Preconception compatible | 1st trimester compatible | Compatible 2nd and 3rd trimester compatible | Lactation compatible | Paternal exposure compatible | Recommendations |
|---|---|---|---|---|---|---|
| Analgesics/anti-inflammatories | NSAIDs and COX-2 inhibitors should be avoided in the 3rd trimester as they can cause closure of the ductus arteriosus (avoid use after week 32) | |||||
| Paracetamol | Yes | Yes | Yes | Yes | Yes | |
| NSAIDs | Yes | Yes | Yes/No | Yes | Yes | |
| COX-2 inhibitors | No | No | No | No | Yes | |
| Corticosteroids | Corticosteroids should be used at the lowest possible dose. The use of high doses has been associated with cataracts, renal failure, and infections. Betamethasone and dexamethasone are used in women at risk of preterm birth | |||||
| Prednisone | Yes | Yes | Yes | Yes | Yes | |
| Methylprednisolone | Yes | Yes | Yes | Yes | Yes | |
| Betamethasone | No | No | No/Yes | No | Yes | |
| Dexamethasone | No | No | No/Yes | No | Yes | |
| Bisphosphonates | Nd | Nd | Nd | Nd | Nd | It is recommended that they be stopped at least 6 months before |
| Antimalarials | Yes | Yes | Yes | Yes | Yes | Although the data are limited, their use is considered safe, preferably hydroxychloroquine |
| DMARDs/Immunosuppressants | Both MTX and LFN must be discontinued at least 3 months in advance. LFN should be washed out with cholestyramine. MMF should be stopped at least 6 weeks in advance. For CyA and tacrolimus, blood pressure, renal function, and drug levels should be monitored | |||||
| Methotrexate (MTX) | No | No | No | No | Yesa | |
| Leflunomide (LFN) | No | No | No | No | Yesa | |
| Azathioprine | Yes | Yes | Yes | Yes | Yes | |
| Mycophenolate (MMF) | No | No | No | No | Yesa | |
| Cyclophosphamide (CFM) | No | No | No | No | No | |
| Cyclosporine (CyA) | Yes | Yes | Yes | Yesa | Yesa | |
| Tacrolimus | Yes | Yes | Yes | Yesa | Yesa | |
| I.v. immunoglobulins | Yes | Yes | Yes | Yes | Yesa | Consider thrombotic prophylaxis |
| Biologics | Although there are no solid data, unintended exposure during the first trimester to these biologics is unlikely to be harmfulRTX will be discontinued at least 6–12 months beforehandCertolizumab pegol is the first biologic that, based on specific studies during pregnancy and lactation as well as registry data, has included amendments in its datasheet on its safety during pregnancy and lactation. Adalimumab has also recently included amendments in its data sheet regarding its safety during pregnancy and lactationUse of TNF antagonists in the third trimester has been associated with an increase in infections in the new-born during the first year | |||||
| Belimumab | No | No | No | No | Nd | |
| Rituximab (RTX) | No | No | No | Nd | Yes | |
| Abatacept | No | No | No | No | Nd | |
| TNF antagonists | ||||||
| Certolizumab pegol | Yes | Yes | Yes/No | Yes | Yes | |
| Adalimumab | Yes | Yes | Yes/No | Yes | Yes | |
| Other | Yes | Yes | Yes/No | Yes | Yes | |
| Anti-platelet/anticoagulants | Clopidogrel should be discontinued at least one week before delivery.Coumarins are teratogenic between weeks 6 and 12 of pregnancy | |||||
| ASA (≤100mg) | Yes | Yes | Yes | Yes | Yes | |
| Clopidogrel | Yes | Yes | Yes | Nd | Yes | |
| Lmw heparin | Yes | Yes | Yes | Yes | Yes | |
| Coumarins | Yes | Yes/No | Yes | Yes | Yes | |
| Rivaroxaban | No | No | No | No | Nd | |
| Anti-HT agents | The use of labetalol is preferably recommended, and atenolol should be avoided because of the risk of restricted foetal growth. Atenolol should also be used with caution during lactation | |||||
| Methyldopa | Yes | Yes | Yes | Yes | Yes | |
| Betablocker | Yes | Yes | Yes | Yes | Yes | |
| Nifedipine | Yes | Yes | Yes | Yes | Yes | |
| Hydralazine | Yes | Yes | Yes | Yes | Yes | |
| Hydrochlorothiazide | Yes | Yes | Yes | Yes | Yes | |
| ACEi/ARB | No | No | No | Yes/No | Yes | |
NSAIDs: non-steroidal anti-inflammatory drugs; ASA: acetylsalicylic acid at anti-platelet doses; anti-HT: antihypertensive; ARB: angiotensin receptor antagonists; lmw: low molecular weight; ACEi: angiotensin-converting enzyme inhibitors; DMARD: disease-modifying anti-rheumatic drugs; nd: no data; TNF: tumour necrosis factor.
This research study received no specific support from public sector or commercial sector agencies or non-profit organisations.
Conflict of interestsG. Espinosa: no conflict of interest to declare.
M. Galindo-Izquierdo: no conflict of interest to declare.
B. Marcos Puig: no conflict of interest to declare.
M. Casellas Caro: no conflict of interest to declare.
P. Delgado Beltrán: no conflict of interest to declare.
J.A. Martínez López: no conflict of interest to declare.
N. Martínez Sánchez: no conflict of interest to declare.
A. Robles-Marhuenda: no conflict of interest to declare.
E. Rodríguez Almaraz: has received funding for presentations, courses and conferences: Novartis, Roche, GSK, Menarini, Grünenthal, Abbvie, UCB and Lilly, and research grants: MSD.
L. Sáez-Comet: no conflict of interest to declare.
A. Ugarte Núñez: no conflict of interest to declare.
P. Vela-Casasempere: no conflict of interest to declare.
J.L. Bartha: no conflict of interest to declare.
G. Ruiz-Irastorza: no conflict of interest to declare.
V.M. Martínez-Taboada: has received funding from Roche for an independent research project, and from Sanofi for presentations.
Please cite this article as: Espinosa G, Galindo-Izquierdo M, Marcos Puig B, Casellas Caro M, Delgado Beltrán P, Martínez López JA, et al. Control del embarazo en pacientes con lupus eritematoso sistémico y síndrome antifosfolípido. Parte 1: Infertilidad, preservación ovárica y valoración preconcepcional. Documento de consenso de la Sociedad Española de Ginecología y Obstetricia (SEGO), Sociedad Española de Medicina Interna (SEMI) y Sociedad Española de Reumatología (SER). Reumatol Clin. 2021;17:61–66.
The full text is available as additional material in Appendix A.