DFS70 ANAs have attracted interest due to their frequency in individuals with no clinical evidence of systemic autoimmune rheumatic disease (SARD), groups with genetic risk for rheumatoid arthritis (RA) were not assessed.
ObjectiveTo determine the frequency of ANA and DFS70 ANA in blood relatives (BR) of people with RA compared to patients with early RA (ERA), and control individuals, and its association with health status.
MethodologyA cross-sectional study with an analytical component. Sixty ERA patients, 60 BR and 120 control individuals paired by age and sex were studied. Hep2-ANA and DFS70 ANA were studied. The absolute and relative frequencies and associations were established using logistic regression models, with a significance level of 95%
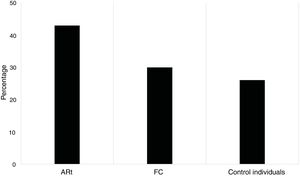
Results43% ANA in ERA, 30% in BR, and 25%-8% in control individuals 1:80. The fine dense granular pattern based on conventional Hep2 was found in 12%-9% of the positive samples, and 1.66% of the total samples. There was no detection of DFS70 ANAs in patients with ERA. In ERA there was an association between the presence of ANA and inflamed joints (P = .02), CRP (P = .01), DAS28CRP (P = .03) and HAQ (P = .04). There was an association between ANA and elevated CRP (P = .05) in the BR. In the control individuals, there was an association between ANA and painful joints (P = .02). In DFS70 ANA individuals we observed an association between a normal ESR P = .032, BR (-), P = .044 and absence of painful joints, p = .039
ConclusionsThe frequency of DFS70 ANA in the groups studied was low, none of the patients with ERA was positive. The presence of DFS70 ANA was only confirmed in systemically healthy individuals.
Los ANA/DFS70 han atraído el interés por su frecuencia en individuos sin evidencia clínica de enfermedad reumática sistémica autoinmune (ERAS), no se han evaluado grupos de riesgo genético para artritis reumatoide (AR).
ObjetivoDeterminar la frecuencia de ANA y ANA/DFS70 en familiares consanguíneos (FC) de AR comparada con pacientes con AR temprana (ARt) e individuos control y su asociación con el estado de salud.
MetodologíaEstudio de corte transversal con componente analítico. Análisis de 60 pacientes ARt, 60 FC y 120 individuos control pareados por edad y sexo. Se analizaron ANA-HEp2 y ANA/DFS70. Establecieron las frecuencias absolutas y relativas y asociaciones con modelos de regresión logística, con un nivel de significancia del 95%.
ResultadosANA de 43% en ARt, 30% en FC y 25,8% en individuos control a título 1:80. El patrón granular fino denso por Hep2 convencional se encontró en el 12,9% de las muestras positivas y el 1,66% del total de muestras. En ANA/DFS70 (+) en 1,66% en FC y 2,5% de individuos control, representando el 75% de la muestras positivas y el 1,25% del total de las muestras. No hubo detección de ANAS-DFS70 en pacientes con ARt. En ARt hubo asociación entre la presencia de ANA y articulaciones inflamadas (p = 0,02), PCR (p = 0,01), DAS28PCR (p = 0,03) y HAQ (p = 0,04). Asociación entre ANA y PCR elevada (p = 0,05) en FC. En individuos control entre ANA y articulaciones dolorosas (p = 0,02). En individuos ANA-DFS70 observamos asociación con VSG normal p = 0,032, FR(-), p = 0,044 y ausencia de articulaciones dolorosas, p = 0,039
ConclusionesLa frecuencia de ANA/DFS70 en los grupos estudiados fue baja, ninguno de los pacientes con ARt fue positivo. Se confirma la presencia de ANA/DFS70 solo en individuos sanos sistémicamente.
ANA presence has been a cause of concern both to clinicians and patients due to the possibility of having or being at risk of developing some type of systemic autoimmune disease. This is even more possible if first-degree blood relatives are affected. Generally both clinical and preclinical control and follow-up is indicated.
During the 12th international workshop on autoantibodies and autoimmunity, the need to establish a consensus was put forward for the naming of the different ANA patterns observed using indirect immunofluorescence (IIF).1 In 2015 the first consensus was published where it was agreed that the description of the ANA patterns was divided into 3 large groups: nuclear, cytoplasmic and myotic, with each of them in turn divided into more specific patterns. In particular the speckled subtype, which also includes a pattern called “dense fine speckled” (AC-2),1–3 the target of which is usually the 70 kDa (DFS70 protein), commonly known as the growth factor derived from the crystalline epithelium p75 (LEDGFp75).4
The anti DFS70/LEDGFp75 (ANA/DFS70) antibodies recently attracted interest for their relative frequency in patients with positive ANA with no clinical evidence of systemic autoimmune rheumatic disease (SARD). Their presence was mainly documented in patients with diverse non-rheumatic inflammatory conditions and in “apparently healthy” individuals.5–11 These antibodies are present in low frequency in patients with SARD.5,8–11
When the anti-DFS70 are present in patients with SARD they are usually accompanied by specific auto antibodies.9–11
This has led to the hypothesis that ANA/DFS70 may be useful markers to exclude the SARD diagnosis and an algorithm for their clinical usefulness has been developed.7,10,11 Reports on the prevalence of ANA/DFS70 in the literature mostly include the Asian, European and North American population,7 and are low on details regarding the Spanish and Latin American population. Since the 1980′s Dr. Graciela Alarcón reported that due to the fact that in Latin American countries approximately half of the population belongs to the group less than 15 years of age, there are differences in relative frequency of rheumatic diseases. Other contributing factors, such as the ethnic composition of the population groups and the sociocultural and environmental characteristics inherent in their condition as developing countries.12 Recently it has been found that the prevalence of RA is higher than that commonly reported, lower for fibromyalgia and gout and similar for osteoarthritis, systemic lupus erythematosus and spondiloarthritis.13
One of the studies undertaken in the Mexican population found there was a frequency of 17% in the healthy population and very low in individuals suspected of having SARD.14 The only study conducted in Colombia,15 did not include the population at risk (relatives) and the results were not in concordance with the findings reported in other populations. It did not include patients with RA in early phases and their relatives. Our study proposal and results are therefore novel. The methodology in the previous study mentioned only included detection of the protein DFS70 without any prior evaluation of ANA by IIF The aim of our study was to determine the frequency of ANA and ANA/DFS70 in BR of patients with RA, compared with patients with ERA and controls, and their association with health status.
Materials and methodsCross-sectional study with analytical component. The study included 240 individuals: 60 patients with ERA, 60 first and second degree BR and 120 healthy controls paired by age and gender for each group, taken from 2 rheumatology centres. This was convenience sampling.
Inclusion criteria of the patients with RA were patients aged between 18–65 years who fulfilled the ACR/EULAR 2010 classification criteria with under 2 years evolution16 and who had received conventional treatment. The BR group was taken randomly from a base of institutional data. Sixty people were included who came from 50 families represented by one individual, 9 families formed by 2 individuals (siblings) and one by 3 individuals (siblings). Forty two were first-degree BR (34 children of mothers with RA, 8 of fathers with RA) and 18 were second-degree BR (siblings of patients with RA) in accordance with the EULAR recommendations (individuals at genetic risk of RA).17 The control individuals were people who lived under working or environmental circumstances which were similar to those of the patients. The exclusion criteria were another autoimmune disease, infectious disease, tumours, diabetes, antibiotic treatment, pregnancy or breastfeeding. Everyone accepted and signed their informed consent, approved by the hospital research and ethics committee, HMC 2017-057.
A complete medical history was obtained relating to RA. The RA disease activity was measured by simplified disease activity indexes (SDAI), the Disease Activity Score 28 (DAS28), Routine assessment of patient index data 3 (RAPID3) and multidimensional health assessment questionnaire (MDHAQ), created by rheumatologists.
High sensitivity C-reactive protein levels were assessed through chemiluminescence (Immulite 1000, Siemmens®), erythrocyte sedimentation rate (ESR) by fluorometry (Test 1 TH L Ali FAX®), anti-cyclic citrullinated antipeptide antibodies (anti-CCP) with the enzymatic method (Quanta lite® CCP 3.1 IgG / IgA, INOVA Diagnostics) and rheumatoid factor (RF) by turbidimetry using Spinreact®).
ANA determination was performed using the IIF technique, with the saline reacting on the plates with the HEp-2 and HEp-2-DFS70 REF 1108 substrate, Autoantibody test System IMCO Diagnostics®. Knocked out, for the psip gene which prevents binding sites to the of 70 kd protein recognised by these autoantibodies. These modified cells are capable of recognising all the other autoantibodies except the DFS70.18 An initial dilution of 1/80 to the final rate was made. The samples with fluorescence was positive with the dense fine speckled stain pattern in the nucleoplasm of the cellular interphase, typically excluding the nucleoli with a shiny stain of chromosomes in the mytotic cellular phase. Each test was run for its respective positive and negative controls.
Absolute and relative frequencies were established for each variable and associations were established with logistic bi and multinomial regression models between parameters of clinical and autoantibody parameters. The statistical STATA package version 13.0 for Windows was used, with all analysis performed with a significance level of 95%.
ResultsDemographic and clinical characteristics of patients with rheumatoid arthritis and relatives with rheumatoid arthritisThe ERA group presented with a mean age of 47.7, in a range between 19 and 64 years (standard deviation, SD: 10.9 years), a mean weight of 65.2 kg, (SD: 16.05 kg), an average height of 1 m 60.0 cm (SD: .08) and a mean BMI of 25.2 (minimum of 15.6 and maximum of 40.8) and SD of 5.18. Eighty one point six per cent (49/60) of the patients were female and 53.3% of the patients (32/60) had comorbidities (Table 1) Only 5% (3/60) smoked and 33.3% (20/60) had smoked at some time in their life. Out of the total patients, 16.6% (10/60) said they were passive smokers.
Demographic characteristics of the study groups.
| Characteristics | ERA | RA control Individuals | BR | RE control Individuals |
|---|---|---|---|---|
| Sex(F) (%) | 81.6 | 81.6 | 76.6 | 75 |
| Age (years)a | 47.7 | 47.6 | 38.5 | 38.6 |
| weight (kgs)a | 65.2 | 63.0 | 65.2 | 62.3 |
| height (mts)a | 1.60 | 1.59 | 1.62 | 1.61 |
| BMIa | 25.2 | 24.6 | 24.5 | 23.7 |
ERA: early rheumatoid arthritis; BR: first degree blood relative of patients with RA.
Regarding economic activity, the patients were most commonly classified as employed, at 41.6%, followed by homemakers at 33.3%.
80% 48/60) of them received standard therapy, and of the 48 patients who received pharmacological treatment, 93.7% (45/48) took methotrexate combined with another disease modifying anti-rheumatoid drug (DMARD). 20% (12/60) had not received pharmacological treatment. 75% of patients presented with more than one painful joint and only 25% stated they had no joint pain. Over half (51.6%) of patients presented with between 1 and 5 painful joints at the time of evaluation. 61.6% of patients had a DAS 28 above 3.2 followed by 30% with lower or equal to 2.6 and 8.4% with between 2.7 and 3.2. Based on the visual analogue scale, it was found that 76.7% of patients suffered from pain. Furthermore, 68.4% of patients according to the SDAI scale were found to be in moderate activity, followed by 26.6% in low activity.
60 individuals who were BR of patients with RA were included in the study. They had a mean age of 38.5 years, in an age range of between 18 and 69 years (SD: 11.8 years), a mean weight of 65.2 kg (SD: 12.27 kg), a mean height of 1 metre 62 cm (SD: .08) and a BMI mean of 24.5 (minimum of 17.6 and maximum of 32) for a SD of 3.34. Seventy six point six percent (46/60) of the subjects were female and 56.7% (34/60) had no comorbidities (Table 1).
Only 5% (3/60) smoked and 15% (9/60) had smoked at some time in their life. Out of the total of BR evaluated, 11.6% (7/60) said they were passive smokers. Regarding economic activity, individuals were most commonly classified as employed, at 66.6%, followed by people who were home makers and those who were self-employed, at 13.3% each. With regards to the type of home, 34 out of the 60 subjects stated they lived in their own house (56.7%), 14 in communal housing and 12 in rented accommodation (23.3% and 20%). Regarding educational level, 65% had been to university.
With regards to joint evaluation, 60% (36/60) presented with no painful joints and 40% (24/60) stated they had more than one painful joint. It was found that a third of individuals presented with between 1 and 5 painful joints and 3.3% with more than this. 83.3% of BR did not present with any joint inflammation (Table 2).
Demographic and clinical characteristics of controlsThe previously described groups were paired in age and gender with health controls. Sixty individuals were included as control individuals of the patients with ERA. They had a mean age of 47.6 years, in an age range of between 19 and 66 years (SD, 11.2 years), a mean weight of 63.04 kg (SD, 10.26 kg), a mean height of 1 m 59 cm (SD, .07) and a mean BMI of 24.66 (minimum 17.9 and maximum 31.6) for a SD of 3.10. Eighty one point six per cent (49/60) of patients were female (Table 1) and 50% of them (30/60) had comorbidities.
The control individuals of the BR of patients with RA comprised 60 individuals with a mean age of 38.6 years, an age range between 18 and 71 years (SD, 12.0 years), a mean weight of 62.3 kg (SD, 12.33 kg), a mean height of 1 m 61 cm (SD: .08) and a mean BMI of 23.76 (minimum 17.5 and maximum 38.7) for a SD of 3.53. Seventy five per cent (45/60) of patients were female and 36.7% of them (22/60) had comorbidities.
Clinically, the total group of control individuals had no painful joints in 72.5% (87/120) and 27.5% (33/120) had more than one painful joint. On classifying the number of painful joints it was found that a fifth of the individuals presented with between 1 and 5 painful joints and 5% with a number above that. 92.5% of the controls did not present with any joint inflammation.
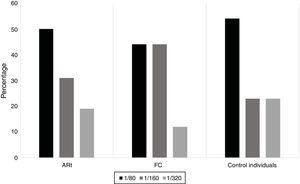
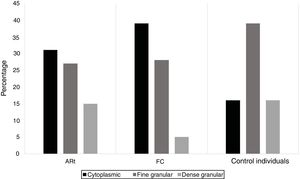
When characterising the ANA profile in patients with ERA, BR and individuals control, it was found that 43% of patients with ERA tested positive for ANA-IIF, 30% in BR and 25.8% in controls (Fig. 1). For all study groups there was a predominance of low rates (1/80) (Fig. 2). The most common patters were cytoplasmic, fine speckled and dense speckled with variable frequencies in the different study groups and the cytoplasmic pattern being most common among the individuals with ERA and BR and the fine speckled pattern most common among the control individuals (Fig. 3).
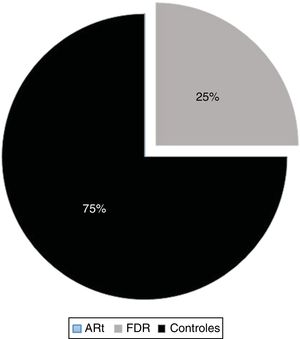
The ANA/DFS70 pattern by conventional IIF was found in 6.6% (5/75) of the sample reported as positive and only in 2.1% (5/240) of the total samples (Table 3). In modified substrate HEp-2 the frequency of the dense fine speckled pattern was 1.66% (n = 4/240) in the total group assessed including ERA, BR and control individuals. In BR it was 1.66% (1/60) and 2.5% (3/120) in control individuals, representing 75% of the positive samples in the group of control individuals (3/4) for this pattern and 1.25% of the total samples (n = 3/240). We did not identify this pattern in patients with ERA (Table 4 and Fig. 4).
Percentage distribution of dense fine speckled pattern by positive ANA-IIF in HEp-2 substrate by study groups.
| Study group | Positive ANA IIF AC-2 (%) | n (%) | % Total samples |
|---|---|---|---|
| ERA | 0/18(0) | 0 (0) | 0 |
| F | 1/26(3.8) | 1 (1.66) | .41 |
| Controls | 4/31(12.9) | 4 (3.33) | 1.66 |
A2 : Dense fine speckled pattern; BR: First degree blood relative of patients with RA; ERA: Early Rheumatoid Arthritis.
Percentage distribution of the dense fine speckled pattern by ANA-DFS70 in modifieda HEp-2 by study groups.
| Study group | Positive ANA IIF-DFS70 (%) | n (%) | % Total sample |
|---|---|---|---|
| ERA | 0/4(0) | 0 (0) | 0 |
| BR | 1/4(25) | 1 (1,66) | .41 |
| Healthy controls | 3/4(75) | 3 (2,5) | 1.25 |
BR: First degree blood relative of patients with RA; ERA: Early Rheumatoid Arthritis.
The individuals who tested positive for ANA-DFS70 in general were women, with a basic primary school educational level. They had been diagnosed with a periodontal disease as comorbidity, an average height of 1.56 m, weight of 58 kg and BMI of 23.83. They had no history of disease or tobacco habit. ESR, CRP, RF and anti-CCp values tested normal and negative. They did not present with any painful or inflamed joint.
To determine the associations between variables 12 logistic regression models were designed for the dependent bi and multinomial qualitative variables, obtaining the following association of greater clinical relevance.
Fifty per cent of the total ANA samples showed rates of 1/160 and 50% higher or equal to 1/320. In patients with ERA it was observed that there was a relationship between the ANA-IIF variable with regard to the gender variable (female) with P = .037, and a major trend was observed between ANA-IIF and inflamed joints with a p = .084 and in the group of BR an association with positive values of CRP (P = .05).
The group of control individuals for BR had an association between ANA-IIF and painful joints (P = .014). In the control individuals for ERA, we observed a relationship between ANA-IIF and tobacco consumption with a P = .001 and disability index with a value of P = .044. A tendency to present with inflamed joints was observed (P = .071).
In the positive ANA-DFS70 individuals we observed an association with normal ESR with a P = .032, negative RF with a P = .044 and absence of painful joints with a P = .039.
DiscussionThe ANA test is commonly used in the lab to determine the presence of any type of autoimmune response when there is suspected clinical symptoms.2,3
Studies performed5,8,11 in recent years found a frequency of 7% to 20% of positivity for ANA between “apparently healthy” individuals; with a dense fine speckled pattern; 5-year follow-ups of these individuals showed no evidence of the development of autoimmune disease despite presenting in some cases with raised rates of ANA/DFS70, reporting frequencies of SARD as low as .45% when they presented alone.10
The presence of ANA in a healthy individual and in BR of patients with SARD generally requires clinical and paraclinical follow-up aimed at early diagnosis of SARD. The importance here is that it may cover the inclusion of this marker to rule out autoimmune disease.11
Researchers have reported an increase in the prevalence of autoimmune disorders between the relatives of patients with RA, idiopathic inflammatory myopathies, insulin-dependent diabetes mellitus and systemic lupus erythematosus, among others.19,20 From here is the interest in including analysis of the determination of the ANA DFS70 in populations at risk such as the BR of patients with AR.
Reports on the prevalence of ANA/DFS70 in the literature include mainly the Asian, European and North American population.5–7 Few data exist on the Hispanic and Latin American population. Based on this, the general aim was to determine the frequency of ANA-IIF and ANA/DFS70 in BR, patients with ERA compared with systemically healthy control individuals, and to effect clinical characterisation of individual with positive autoantibodies and evaluate their possible association with health status.
As was to be expected, the majority of subjects were middle aged females. When pairing up the control group individuals by age and gender although the majority had no major comorbidity or associated autoimmunity, the present or past history of a tobacco habit was striking in a third of this population.
The frequency of positivity for ANA-IIF was close to 31%, being relatively similar to that reported in the literature where standard frequencies have been described as fluctuating between 20%21 and 25%22 in the general population. Performing analysis by group discrimination, the positivity of frequency for ANA-IIF, using as a cut-off point 1/80, was higher in the ERA group where frequencies of up to 43% were reached. This concurs with the literature.23 To be noted too is the positivity for ANA-IIF in a third of the BR, which has also been reported in other BR groups of autoimmune diseases and of RA, suggesting that in our population the genetic conditioning could have a disease load which is higher than the expositional factor.24–26
In all population groups there was a predominance of low rates, with the most frequent being rates of 1/80, and this is in keeping with that reported by Wanatabe et al.21 in their study on the Asian population and Mariz10 where they reported frequencies of this rate close to 54% and that reported in the healthy Mexican population.27,28
Regarding the ANA-IIF patterns, the following 3 predominated; 2 speckled and 1 cytoplasmic, with notable predominance of the cytoplasmic patterns in patients with ERA and BR. In contrast, in control individuals, similarly to that described by Mariz et al.,10 the most frequent pattern was the fine nuclear speckled pattern, suggesting again that in our population, the recognized epitopes between healthy individuals and RA traditionally involved in the development of autoimmune disease are different.
The presence of ANA-IIF was associated with females in RA patients, in keeping with that described in the literature, where for this disease it was estimated that there was a M:W ratio of 1:3. Furthermore, a relationship was found between the exposure to tobacco for control individuals of RA which has been described within the autoimmunity development.29,30
From the above it may be considered that the frequency of positivity for ANA-IIF in healthy individuals with no blood relationship, environmental exposure would play a leading role in contrast to that shown in healthy individuals with a blood relationship, where genetic weight could be more important than environmental.31
In patients with ERA, an association was found between ANA-IIF and the presence of inflamed joints, which contrasts with that described in the literature that generally does not describe the association in RA between the positivity of ANA-IIF and inflammatory joint activity. In contrast there was no association with positivity for anti-CCp.32
In BR a major trend was found between the presence of ANA with elevated CRP levels and in the control individuals of this group with the presence of painful joints. Although additional studies would be required, it could be thought that the presence of ANA in healthy individuals with genetic risk could be associated with subclinical joint damage from the activation of joint remodelling pathways.33
In general terms the dense fine speckled pattern by conventional HEp-2 substrate IIF was found at low frequency in all study groups, with the highest being in the control group where it represented 12.9% of the samples reported as positive and 1.66% of the total samples. There was a lower frequency than expected in both healthy individuals and in those with RA10 but it was in keeping with that reported by the Gómez-Puerta17 group in the only previous study conducted in Colombia and synchronic with the low frequency of this pattern in patients with RA.
As with that reported by conventional IIF, in our study the dense fine speckled pattern through the IIF modified HEp-2 substrate technique was found in low frequency in all study groups, being higher in the group of control individuals where it represented 75% of the reported samples as positive and 1.25% of the total samples. In the diverse studies performed variables frequencies were reported for positivity of ANA/DFS70, the majority of them performed in conventional substrates.16,21 Of major importance was the fact that it was observed that ANA-DFS70 positive individuals were associated with an absence of painful joints which allowed them in future studies to determine the population at risk of developing joint compromise.
The DFS70 pattern comes from the autoantibodies which bind the protein expressed ubiquitously by the LEDGF product of the p75 or psip1 gene which is often observed during the detection of ANA through IIF-HEp-2, and considered the most important antigen against which these antibodies are directed34; using this substrate we find there is a frequency of positivity for ANA-DFS70 of 5% among the ANA-IIF positive individuals, of 1.6% of the total sample studied and of 2.5% in healthy control individuals. There are no studies in the Colombian population that have used this technique, since the only one conducted solely made the determination directly of the protein by chemioluminiscense,17 and from there we deduce that no previous data exist for the Colombian population regarding rates and patterns.
Evaluation of monospecific anti-DFS70 antibodies is clinically essential and challenging using traditional HEp-2 IIF.35 The prevalence of anti-DFS70 depends on the assay applied and the level of clinical care.36,37 Although the aim of this study was not to compare results in the detection of the DFS70 pattern using conventional ANAS IIF compared with its modified version, the results obtained in this study show there are differences in the readings. These concur with the study that supports the hypothesis that the lack of standardisation in the IIF kits, together with the subjectivity of user interpretation, among other factors, together reduce the application of the new current algorithm for the detection of individuals with suspected connective tissue diseases.35
Despite the low ANA/DFS70 frequency in both conventional substrate and modified substrate, this patent was only observed in healthy BR individuals for RA and control individuals without any genetic autoimmunity relationship, which confirms its positivity in healthy individuals. Corroborating also by association with an absence of clinical markers such as negative ESR and RF and absence of painful joints, suggesting a status of well-being in the individuals who express positive ANA-DFS70.11
ConclusionsThe frequency of ANA/DFS70 in the groups studied was low, but none of the patients with ERA who were included in the study tested positive. The presence of ANA/DFS70 was confirmed in systemically healthy individuals with or without genetic risk factors. The associations with clinical and serological variables found require further study to demonstrate their role within the diagnostic algorithms for this entity.
FinancingThis protocol was supported by IMMCO Diagnostic, Dizar Ltda and the Institute of Science, Technology and Innovation, Francisco José de Caldas-COLCIENCIAS (N.o 130865740792-2014).
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Ms. Ángela Arias Arias for her collaboration in the editing of this manuscript.
Please cite this article as: Romero-Álvarez V, Acero-Molina DA, Beltrán-Ostos A, Bello-Gualteros JM, Romero-Sánchez C. Frecuencia de ANA/DFS70 en familiares de pacientes con artritis reumatoide comparados con pacientes con artritis reumatoide y población sana y su asociación con el estado de salud. Reumatol Clin. 2021;17:67–73.