Cardiac involvement in Takayasu arteritis (TA) is the major cause of morbidity and mortality. Cardiovascular magnetic resonance (CMR) is an excellent modality for the assessment of myocardial involvement. Studies have shown myocardial involvement in 25%–27% of patients.
ObjectivesTo evaluate the prevalence and pattern of myocardial involvement in TA on CMR. We also evaluated any correlation between CMR changes and disease activity score (ITAS 2010 and ITAS-A) assessed at the time of CMR.
MethodsPatients classified as Takayasu arteritis according to Sharma et al. criteria were enrolled in the study. Demographic, clinical, and laboratory data were documented in the predesigned proforma. CMR was performed on a dedicated cardiac 3Tesla MR machine. Disease activity was recorded by ITAS2010 and ITAS-A.
ResultsA total of 37 TA patients were included. Mean (±SD) age was 29±11 years. Female to male ratio was 3:1. Five patients (14%) had myocardial involvement on CMR. Two (2/5) had myocarditis and three (3/5) patients had features of ischaemic myocardial fibrosis.
ConclusionThe myocardium is affected in TA, however the prevalence of subclinical myocardial involvement in our study was less (8% vs. 25%–27%) compared to the previous studies. Myocardial involvement trends towards early age of onset, less disease duration, lack of classical risk factors, and more with disease activity.
La afectación cardíaca en la arteritis de Takayasu (AT) es la principal causa de morbmortalidad. La resonancia magnética cardiovascular (RMC) es una modalidad excelente para la evaluación de la afectación miocárdica. Los estudios han demostrado afectación del miocardio en el 25-27% de los pacientes.
ObjetivosEvaluar la prevalencia y patrón de afectación miocárdica en AT en RMC. También se evaluó cualquier correlación entre los cambios de RMC y la puntuación de actividad de la enfermedad (ITAS 2010 e ITAS-A) evaluada en el momento de la RMC.
MétodosPacientes clasificados como arteritis de Takayasu según los criterios de Sharma et al. se inscribieron en el estudio. Los datos demográficos, clínicos y de laboratorio se documentaron en el formulario prediseñado. La RMC se realizó en una máquina de RM cardíaca de 3 Tesla dedicada. La actividad de la enfermedad fue registrada por ITAS 2010 e ITAS-A.
ResultadosSe incluyeron un total de 37 pacientes con AT. La edad media (±DE) fue de 29±11 años. La proporción de mujeres a hombres fue de 3:1. Cinco pacientes (14%) tenían afectación miocárdica en la RMC. Dos (2/5) tenían miocarditis y 3 (3/5) pacientes tenían características de fibrosis miocárdica isquémica.
ConclusiónEl miocardio es afectado en la AT, sin embargo, la prevalencia de afectación miocárdica subclínica en nuestro estudio fue menor (8% vs. 25-27%) en comparación con los estudios previos. La afectación miocárdica tiende hacia una edad de inicio temprana, menor duración de la enfermedad, falta de factores de riesgo clásicos y más con la actividad de la enfermedad.
Takayasu arteritis (TA) is a large vessel vasculitis of unknown aetiology affecting mainly aorta and its major branches.1 Nearly 50% of patients with TA can have cardiac involvement during their course of the disease, and is the major cause of morbidity and mortality.2 Myocardial involvement may be due to myocarditis or myocardial ischaemia secondary to coronary arteries involvement manifesting as myocardial fibrosis, with reported range from 7% to 83% of cases in different studies.3–7 Endomyocardial biopsy remains the gold standard for the diagnosis of myocarditis. However, its being invasive, relative lack of sensitivity, combined with limited availability of the experienced operators, equipment and cardiovascular pathology resources and procedure related complications limits its use.8 CMR is an excellent non-invasive modality which is more accurate and reproducible technique for assessment of cardiac morphology and function than any other method.9,10 Myocardial fibrosis,11,12 indicative of remote myocardial disease, can only be diagnosed by CMR.
Assessment of disease activity in TA is a less defined and is a challenge for the clinicians. Indian Takayasu clinical Activity Score (ITAS2010) is a composite score of disease activity developed in India, and its version incorporating serum acute phase reactants (ESR and CRP) is ITAS-A. It is better than physician global assessment (PGA) in term of comprehensiveness and the inter-rater agreement.13
In the context of limited literature on involvement of myocardium in TA, this study was planned to evaluate prevalence of subclinical myocardial involvement in TA by CMR and its correlation with disease activity score (ITAS 2010 and ITAS-A).
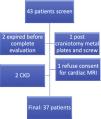
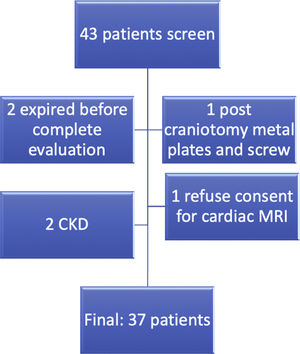
MethodsA total of 37 patients classified as TA,14 were included after informed consent (Fig. 1). Demographic, clinical and laboratory data were recorded in the predesigned proforma. All patients were evaluated by transthoracic echocardiography in standard parasternal, apical, and sub-xiphoidal view by an experienced cardiologist (AB). Disease activity was recorded by ITAS2010 and ITAS-A (AC).
CMR was done on 3.0Tesla Philips Insignia (Philips healthcare, Best, The Netherlands) system. Evaluation has been done by a single expert (MS) blinded to the clinical data on a dedicated work station (Philips Intel space portal).
Details of CMR protocol as followsCMR was done on 3Tesla Philips Insignia system loaded with dedicated cardiac MR software post-processing of the data was done on a dedicated Philips intel space portal. Scan protocol was as follows:
- 1.
Axial stack (black blood from lung apices to diaphragm and bright blood).
- 2.
Cine SSFP (study state free precision): sequence in four planes (single slice four chamber, single slice two chamber, single slice three chamber, short axis stack from AV valves to ventricular apex) slice thickness 8mm with a gap of 25%.
- 3.
Myocardial oedema sensitive sequences (T2 STIR sequences in three planes single slice four chamber, single slice two chamber, and at least 3 slices through left ventricular base, mid-cavity, and apex).
- 4.
Early gadolinium enhancement images (immediately after injection of I/V gadolinium @ 0.02mmol/kg).
- 5.
Look-Locker sequence for estimation of myocardial nulling time.
- 6.
Late gadolinium enhancement after 10min of I/V contrast.
Evaluation has been done by a single expert (MS) blinded to the clinical data, with the help of dedicated Philips software for CMR evaluation. Reporting has been done on three broad category – first. Thoracic anatomy, which includes structural details of the aorta and pulmonary arteries. Anatomy of pericardium also has been provided under this section. The second part is resting cardiac function, which includes all the four chambers diameter, ventricular systolic functions (LV and RV), valve (mitral and tricuspid) anatomy and function. The third component was tissue characterization.
Oedema: Myocardial oedema appears as a high signal intensity on STIR (short tau inverse recovery sequence was used for evaluation of myocardial oedema as it suppress the background tissue and accentuating detection of myocardial oedema). Presence of oedema in the absence of late gadolinium enhancement (LGE) is suggestive of reversible myocardial injury. It is recommended to consider only those areas with at least ten adjacent pixels with high signal intensity as significant.
Early gadolinium enhancement (EGE): EGE has been defined as increase normalized gadolinium accumulation in the myocardium in the early washout period. Irregular breathing and arrhythmia may be a confounding factor.
LGE: The expert has noted visual interpretation of presence or absence along with pattern and location. The pattern and extent of LGE were assessed by both short and long axis view. Myocardial scarring was defined as positive only when it was found to be present in two orthogonal planes. Lake Louise criteria have been used as a comprehensive criterion to identify myocarditis.
Data analysis was done on SPSS-21/MS – Excell software and expressed as mean (standard deviation) for quantitative variables and counts and percentage for the categorical variables. Median was used for skewed data. Proportions were compared by using Chi-square or Fisher's exact test whichever is applicable. For correlation between different variables, Pearson or Spearman correlations were calculated. All statistical tests were two-sided, and a p value ≤0.05 was considered significant in all statistical evaluations. Descriptive statistics have been used for late gadolinium enhancement.
The study was approved by the Institutional Ethics Committee (INT/IEC/2018/001538), and the principles of the 1964 Helsinki declaration were adhered to.
ResultsThe mean age of patients (n=37) was 28.92±10.7 years (range 14–58 years). There were 28 females (75.68%) and 9 males (24.32%) with a female to male ratio of 3.1:1. The mean age of symptom onset was 23.86±8.4 years (range 11–46 years). The median disease duration was 5.1±5.1 years (range 1 year to 27 years). Claudication was the commonest presenting symptoms (89%), followed by easy fatigability (73%), headache (67%), dyspnoea (57%), chest pain (32%), joint pain (19%), and syncope (16%). Details of baseline characteristics provided in Table 1.
Demographic features, clinical profile, and investigations of Takayasu patients.
| Variable | All patients (n=37) |
|---|---|
| Age (years) (SD) | 28.9 (±10.7) |
| Female sex (%) | 28 (75.7) |
| F:M | 3.1:1 |
| Age at onset of symptoms (years) (SD) | 23.9 (±8.4) |
| Disease duration (years) (SD) | 5.1 (±5.1) |
| BMI (kg/m2) (SD) | 23.5 (±4.4) |
| Hypertension (%) | 24 (64.9) |
| Bruit (%) | 28 (75.6) |
| Pulse inequality (%) | 32 (86.5) |
| Murmur (%) | 7 (18.9) |
| Transient ischaemic attack/stroke (%) | 2 (5.4) |
| Smoking (%) | 1 (2.7) |
| Past history of myocardial infraction (%) | 1 (2.7) |
| Hb [g/dl] (SD) | 12.3 (±1.9) |
| TLC [×109] (SD) | 10.8 (±3.4) |
| Platelets [×109] (SD) | 289 (±112) |
| ESR [2–20mm in 1st hour] (SD) | 36 (±17) |
| CRP [<6mg/dl] (IQR) | 22.2 (±22.53) |
| Cr [mg/dl] (SD) | 0.74 (±0.2) |
| Albumin [g/dl] (SD) | 3.9 (±0.5) |
| Cholesterol [mg/dl] (SD) | 178.2 (±48.8) |
| LDL [mg/dl] (SD) | 107.1 (±42.5) |
| HDL [mg/dl] (SD) | 53.08 (±13.7) |
Examination at recruitment revealed unequal pulse in 32/37 (87%) patients, bruit in 28/37 (76%), and hypertension in 24/37 (65%) at recruitment. Two patients (5%) had diabetes with disease control by oral hypoglycaemic agents. Four patients (11%) were hypothyroid, and were under control by oral levothyroxine.
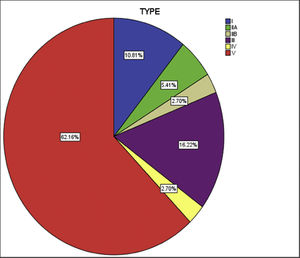
Both left subclavian, and descending thoracic aorta were affected in 28 (75%) patients, the commonest vessels involved in the cohort. The abdominal aorta was the second most common, in 26 (70%) patients. The cohort was classified into six groups as per Numano's angiographic types. Type V was the commonest type present in 23 (62%) patients followed by type III in six (16%), type I in four (10%), type II (combined IIa and IIb) in 3 (8%) and type IV in one (2%) (Fig. 2).
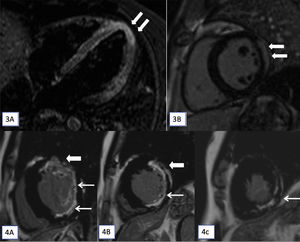
Myocardial involvement on CMR was noted in five patients (n=5/37). Two patients (2/5) had CMR features of myocarditis (Figs. 3 and 4). Three (3/5) had CMR evidence of ischaemic myocardial fibrosis. Details of myocardial involvement and distribution are provided in Table 2.
(3A and 3B): T2-STIR (short tau inversion recovery) cardiac magnetic resonance imaging (CMR) images in horizontal (A) long axis show myocardial hyperintense signal in the apex (arrows). Late gadolinium enhancement (LGE) apical short axis image shows confluent epicardial predominant myocardial enhancement. On cine cardiac MRI imaging only subtle hypokinesis was seen (not shown here). (4A–4C): CMR, LGE images in short axis at base (A), mid cavity (B) and at apex (C) shows transmural enhancement in the anterior segments (thick arrow in A and B) and sub-endocardial in lateral and inferior segments (A and B) and at apex (C) in the left anterior descending and left circumflex territories (7/17 segments).
Summary of pattern of myocardial involvement.
| S. no. | Age/sex | Type of disease | Pattern of myocardial involvement on CMR and relevant ECHO findings | Diagnosis | Disease status (A=Active/I=Inactive)[Treatment] |
|---|---|---|---|---|---|
| P1. | 19/F | V | Hyperintense signal in LV apex on oedema sensitive sequence (STIR). Late gadolinium enhancement (LGE) showed confluent epicardial predominant myocardial enhancement. On cine cardiac MRI imaging only subtle hypokinesis was seen. Echo normal. | Acute myocarditis | A [Methotrexate+oral prednisolone] |
| P2. | 27/M | IV | On CMR, LGE images subendocardial enhancement seen in the apical anteroseptal and inferior walls. No regional wall abnormalities. Echo normal. | Ischaemic fibrosis/scar in RCA territory | I [Oral methotrexate] |
| P3. | 38/M | V | On CMR, LGE – base-transmural enhancement inanterior wall and sub-endocardial enhancement in lateral and inferiorwall; mid cavity-transmural in anterior and inferior and sub-endocardial in lateral walls; and apex subendocardialenhancement. Regional wall motion abnormalities were noted in the involved segments. Echo findings were corroborative with regional wall motion abnormalities in the same segments. | Ischaemic fibrosis/scar in left anterior descending and left circumflex arterial territories | A [Injectable methotrexate+oral prednisolone] |
| P4. | 18/F | V | On CMR, LGE subendocardial enhancement in mid anterior wall. Mild hypokinesis co-localizing to LGE on cine imaging. Echo-corroborative. | Ischaemic fibrosis/scar in left anterior descending artery | I [Oral methotrexate] |
| P5. | 18/F | V | On CMR cine imaging (SSFP) mild hypokinesia of apical lateral wall. LGE-epicardial predominant enhancement in lateral wall. Echo-corroborative | Acute myocarditis | A [Oral steroid+oral methotrexate] |
STIR=short tau inverse recovery; SSFP=steady state free precession; M=male; F=female.
On the evaluation, there were higher percentage of patients in the age group of <18 years, higher CRP value, more patients with active disease (both ITAS2010 and ITAS-A), and less number with conventional risk factors like high cholesterol, LDL, and Framingham risk score (FRHS) although none of those parameters had achieved statistical significance level (Table 3).
Relation of different risk factors with myocardial heart disease.
| Parameters | MHD− (n=32) | MHD+ (n=5) | p |
|---|---|---|---|
| Disease duration >5 yrs | 13 (40.6) | 2 (40) | 0.979 |
| Age at onset <18 yr | 7 (21.9) | 3 (60) | 0.074 |
| BMI>23 | 19 (59.3) | 1 (20) | 0.1 |
| Hb<12gm/dl | 16 (50) | 2 (40) | 0.677 |
| Plt>4.5 lacks | 4 (12.5) | 0 (0) | 0.403 |
| ESR>20 | 28 (87.5) | 4 (80) | 0.648 |
| CRP>10 | 16 (50) | 4 (80) | 0.211 |
| CH>200 | 10 (31.2) | 1 (20) | 0.609 |
| LDL>130 | 9 (28.1) | 0 (0) | 0.173 |
| BNP>125 | 11 (34.4) | 3 (60) | 0.272 |
| Type V | 19 (59) | 4 (80) | 0.376 |
| ITAS2010-Active | 17 (53.1) | 3 (60) | 0.774 |
| ITAS-A-Active | 11 (34.4) | 3 (60) | 0.272 |
| FHRS-intermediate risk | 4 (12.5) | 0 (0) | 0.567 |
Relationship with arterial bed involvement: significant association with right renal artery involvement (p=0.037) and higher but non-significant association with the abdominal aorta (p=0.085) and left renal artery (p=0.079) involvement.
DiscussionMyocardium involvement has been reported in TA3–7; however, there are limited studies on CMR evaluating myocardial involvement in TA.8,9 Myocardium can be affected as myocarditis, ischaemia (due to coronary artery involvement), secondary to valvular disease or reno-vascular hypertension. Up to 50% of patients of TA can have subclinical myocardial inflammation as documented by different histopathological studies.4,17 Endomyocardial biopsy though remains the gold standard, however, CMR is now the modality of choice due to non-invasive imaging without any hazard of ionizing radiation. It also offers better anatomical visualization, less inter-observer variability; more accurate quantitative assessment and tissue characterization based on pattern of myocardial enhancement on late gadolinium enhancement (LGE) images.
Our study enrolled 37 patients with mean age of 28.9±10.7 years compared to 49±14 and 48 (34–61) years, in previous studies on cardiac involvement in TA, evaluated by CMR.5,6 The current study showed a changing trend towards female dominance compared to the previous studies.15 Initial presentation with systemic symptoms like fever and weight loss were a distinct finding compared to the previous studies. Claudication pain (89%) and constitutional symptoms like easy fatigability (70%), weight loss (54%), and fever (51%) were the main symptoms in the current study, whereas systemic symptoms were present only in 16% of cases and hypertension was the commonest in previous Indian studies.16 According to the distribution of vessels type V was the most common variant, consistent with the previous Indian data. But there are substantial changes in the distribution of vessel involvement. In the current study, there is significant proximal migration of vessel involvement compared to previous data.
In our study protocol myocardial involvement was characterized as due to myocarditis, ischaemia or non-specific changes. Two previously reported studies on the same subject reported myocardial involvement but were silent on further characterization.5,6
In current study 14% (5/37) had myocardial involvement as detected by CMR with 2/5 having myocarditis and 3/5 having features of ischaemic myocardial fibrosis secondary to coronary arteries involvement. CMR diagnosed myocardial involvement in two cases (Table 2, P1 and P5), which were missed by echocardiography.
In 13.5% (5/37) patients in our study had CMR abnormality compared to 27% and 26% in the previous studies (Table 4).5,6 Of the three patients, 2 (5%) had LGE displayed typical pattern of myocardial ischaemia (MI), whereas study by Comarmond et al.6 in 22% had LGE distribution typical of MI, and had three times higher association with renovascular hypertension. Compared to the previous studies the current study had lower age of onset, less disease duration and lower number of diabetic patients. These factors may contribute to higher percentage of cardiac MRI abnormalities in previous studies. LGE typical of myocardial fibrosis in CMR can be found in many condition including hypertension.18 The main difference from the earlier studies were mean age, age of onset, and disease duration, all of which were much less in the current study. Other than genetic and ethnic variation, the higher percentage of LGE detected by the previous studies may be a reflection of cumulative damage with increasing age, prolong hypertension and disease duration.
Review of studies on cardiac involvement in TA evaluated by CMR.
| Parameters | Keenan et al.5(2009; n=16) | Comarmond et al.6 (2013; n=27) | Current study (n=37) |
|---|---|---|---|
| Age (mean) | 49 | 48 | 28.9 |
| Female (%) | 100 | 70.4 | 75.7 |
| Age of onset (mean) | NA | 36 | 23.9 |
| Disease duration (mean) | NA | 9 | 5.1 |
| DM (%) | 13 | 14.8 | 5.4 |
| HTN (%) | 56 | 37 | 64.9 |
| Dyslipidaemia (%) | 38 | 33.3 | 35.1 |
| Family h/o CAD (%) | NA | 11.8 | 8.1 |
| Tobacco (%) | 6 | 25.9 | 2.7 |
| Myocardial oedema (%) | 0* | 0* | 2.7 |
| LGE (%) | 27 | 25.9 | 8.1 |
We recognize various limitations of this study. The optimal timing for CMR in TA and effect of treatment on CMR findings has not been addressed. Published literature till date has also not commented on this valid issue. Further large multi-centric studies are thus required for specific answers. Another limitation was that not all the patients had undergone coronary angiography for assessment of coronary arteries.
Prior presentation: The data was presented partially at EULAR e-Congress 2020, as a poster presentation.
Study highlightsThis was the largest cohort of TA evaluated by CMR showed prevalence of subclinical myocardial involvement was much less (8% vs. 25–27%) compared to the previous studies. Myocarditis was not mentioned in the previous studies. Myocardial involvement trend towards early age of onset, less disease duration, lack of classical risk factors, and more with disease activity.
The current study showed a change in demography, presenting clinical features, and distribution of arterial bed involvement in Indian patients with TA.
Echocardiography may miss some cases of myocardial involvement, especially the acute myocarditis which can only be detected by CMR, hence it can miss the window of opportunity of treatment. Judicious use of CMR may help in detecting subclinical myocardial involvement and can alter the treatment outcome.
FundingNone.
Conflicts of interestNone of the study authors have any conflict of interest.