There are controversial results regarding the value of serum IL-8 and TNFα in patients with non-specific low back pain.
This study aimed to compare pro-inflammatory cytokines between patients with non-specific back pain and pain-free controls.
Materials and methodsWe conducted a case–control study including 106 participants: 46 patients with chronic non-specific low back pain (G1) and 60 pain-free controls (G0). The interleukin (IL-)6, IL-8, IL-17, IL-23, IL-22, and Tumor necrosis factor α (TNFα) were measured. We collected demographic and clinical data, including age, gender, low back pain duration and radicular pain. The pain degree was assessed using the Visual Analogic Scale.
ResultsThe mean age was 43.17±8.7 years in G1. Radicular pain was found in 37 cases with a Visual Analogic Scale of 3.03±2.5mm. The magnetic resonance imaging was performed in (G1), showing disk herniation and degenerative disk disease in 54.3% (n=25) and 45.7% of cases (n=21), respectively. The IL-8 was higher in G1 (18.84±44.64 versus 4.34±1.23pg/mL, p:0.033). IL-8 levels correlated with TNFα (0.942, p<10–3), IL-6 (0.490, p=0.011) and Visual Analogic ScaleRadicular-pain (r:0.297, p:0.047).
IL-17 was higher in patients with restricted lumbar spine mobility (9.64±20.77 versus 1.19±2.54pg/mL, p:0.014).
ConclusionsOur results provide evidence that IL-8 and TNFα play a role in low back pain and radicular pain due to disk degeneration or herniation. These findings could potentially be used by future studies to develop new non-specific low back pain therapeutic strategies.
Existen resultados controvertidos en cuanto al valor de la interleucina (IL) 8 y el factor de necrosis tumoral α (TNFα) séricos en pacientes con lumbalgia inespecífica.
Este estudio tuvo como objetivo comparar las citoquinas proinflamatorias entre pacientes con dolor de espalda inespecífico y controles sin dolor.
Materiales y métodosRealizamos un estudio de casos y controles que incluyó a 106 participantes: 46 pacientes con dolor lumbar crónico inespecífico (G1) y 60 controles sin dolor (G0). Se midieron las IL-6, IL-8, IL-17, IL-23, IL-22 y el TNFα. Recopilamos datos demográficos y clínicos, incluidos la edad, el sexo, la duración del dolor lumbar y el dolor radicular. El grado de dolor se evaluó mediante la escala analógica visual.
ResultadosLa edad media fue de 43,17±8,7 años en G1. Se encontró dolor radicular en 37 casos con una escala analógica visual de 3,03±2,5mm. La resonancia magnética se realizó en G1, mostrando hernia discal y enfermedad discal degenerativa en el 54,3% (n=25) y el 45,7% de los casos (n=21), respectivamente. La IL-8 fue mayor en G1 (18,84±44,64 versus 4,34±1,23pg/ml, p=0,033). Los niveles de IL-8 se correlacionaron con TNFα (0,942, p<10−3), IL-6 (0,490, p=0,011) y escala visual analógicadolor radicular (r=0,297, p=0,047).
IL-17 fue mayor en pacientes con movilidad restringida de la columna lumbar (9,64±20,77 versus 1,19±2,54pg/ml, p=0,014).
ConclusionesNuestros resultados proporcionan evidencia de que la IL-8 y el TNFα juegan un papel en el dolor lumbar y en el dolor radicular debido a la degeneración o a hernia discal. Estos hallazgos podrían potencialmente ser utilizados por estudios futuros para desarrollar nuevas estrategias terapéuticas no específicas para el dolor lumbar.
Low back pain (LBP) is a global healthcare concern. Non-specific LBP is the most common form.1 It can be due to several diseases, such as disk disease (intervertebral disk degeneration (IDD) or lumbar disk herniation (LDH)), facet joint osteoarthritis, and spinal stenosis.2
Non-specific LBP is a very common disease that affects 70 to 90% of general population.3 Fifteen per cent of work absenteeism is caused by LBP.3 It can be responsible for disability and restricted lumbar range of motion.4
Thus, a better understanding of the pathophysiology of low back pain is necessary to ensure appropriate care for this potentially disabling disease.
Despite its frequency, LBP's diagnosis remains sometimes difficult and mainly based on the elimination of other spinal diseases.5 LBP pathophysiology is, up to now, unclear.
There are controversial results regarding the role of pro-inflammatory cytokines such as interleukin (IL-)6, IL-8, IL-17, IL-23, IL-22, and Tumor necrosis factor (TNFα) in the physiopathology of non-specific LBP5,6 and its recurrence.7
Besides, several studies showed that pro-inflammatory cytokines contribute to the pathogenesis of IDD and associated pain mechanisms.8,9
This study aimed to compare the value of these pro-inflammatory cytokines between patients with non-specific LBP and pain-free controls.
Material and methodsPatientsWe conducted a case–control study from September 2019 to December 2020 including 106 participants:
- -
G1: 46 consecutive patients with non-specific LBP recruited from the outpatient clinic of the rheumatology department.
- -
G0: 60 pain-free controls recruited from different departments in the Military hospital of Tunis.
Were included in this study patients with chronic non-specific LBP lasting>3 months.
Only patients with magnetic resonance imaging (MRI) showing single LDH or IDD were included in the study. The interpretation of MRIs was performed by a trained radiologist.
Exclusion criteriaNon-inclusions criteria for the two groups were: history of neoplasia, asthma, infections, chronic inflammatory bowel disease, psoriasis, auto-immune disease (rheumatoid arthritis, systemic lupus erythematosus), or steroids treatment.
Patients with LBP treated with surgery were not included.
Were excluded patients with LBP due to spondyloarthritis, infectious spondylodiscitis, or metastasis.
Clinical assessmentWe collected demographic and clinical data, including age, gender, age at disease onset, LBP duration, radicular pain, and therapeutic management.
The pain degree was assessed using the Visual Analogic Scale (VAS). The VAS varied from “no pain” to “worst imaginable pain” (0–100mm).
Lumbar spine mobility was evaluated using Schober's test.10 A restricted lumbar motion was defined as a difference between the measurements in erect and flexion positions lower than 5cm.
Cytokine's measurementThe IL-6, IL-8, IL-17, IL-23, IL-22, and (TNFα) were measured. The blood samples were collected, centrifuged, and frozen at −80°C. IL-6, IL-8, and TNFα were measured using the chemiluminescence method, whereas IL-17, IL-22, and IL-23 levels were measured using the ELISA (Enzyme-linked Immunosorbent Assay).
Statistical analysisStatistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software version 25. We used descriptive statistics to identify the characteristics of the study population. Results were expressed as the mean±standard deviation (SD).
Continuous variables were compared using t-tests for normally distributed variables and the Mann–Whitney U test for non-normally distributed groups. Correlations were tested using the Pearson or Spearman correlation test, depending on data distribution. p-Value<0.05 was considered statistically significant.
To assess the normality of data, we used the Kolmogorov–Smirnov test. Receiver operating characteristic (ROC) curves were used for each cytokine to evaluate their ability to discriminate patients with non-specific LBP from pain-free controls.
Ethical considerationThis study was approved by eight members of the ethics committee of the hospital in May 2019.
All participants were requested to read and sign an informed consent form explaining the aim and process of the study.
ResultClinical and epidemiological characteristics of patientsAll patients were Caucasian. There were 36 men in G1 and 46 in G0. The mean age was 43.17±8.7 years in G1 and 39.56±9.95 years in G0. The mean body mass index (BMI) was 27.31±3.47kg/m2.
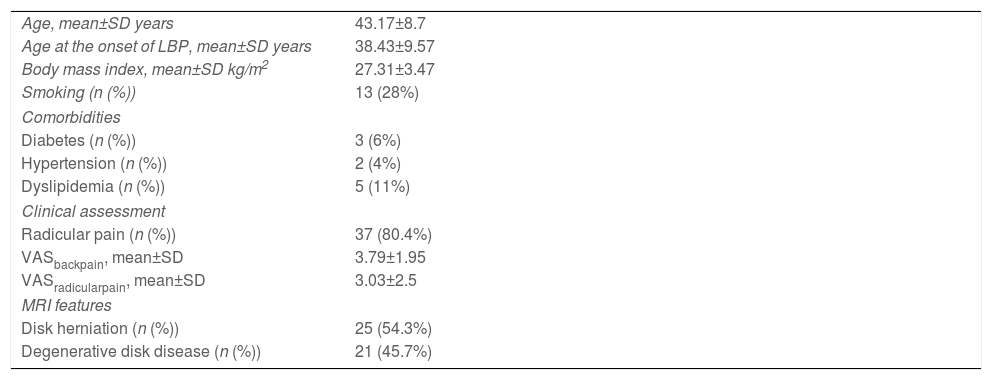
Clinical characteristics of patients with non-specific LBP are summarized in Table 1.
Clinical characteristics of patients.
| Age, mean±SD years | 43.17±8.7 |
| Age at the onset of LBP, mean±SD years | 38.43±9.57 |
| Body mass index, mean±SD kg/m2 | 27.31±3.47 |
| Smoking (n (%)) | 13 (28%) |
| Comorbidities | |
| Diabetes (n (%)) | 3 (6%) |
| Hypertension (n (%)) | 2 (4%) |
| Dyslipidemia (n (%)) | 5 (11%) |
| Clinical assessment | |
| Radicular pain (n (%)) | 37 (80.4%) |
| VASbackpain, mean±SD | 3.79±1.95 |
| VASradicularpain, mean±SD | 3.03±2.5 |
| MRI features | |
| Disk herniation (n (%)) | 25 (54.3%) |
| Degenerative disk disease (n (%)) | 21 (45.7%) |
LBP: low back pain, VAS: Visual Analog Scale, MRI: magnetic resonance imaging.
The mean age at the onset of LBP was 38.43±9.75 years.
The mean duration of LBP was 4.72±3.03 years.
Radicular pain was found in 37 cases with a VAS of 3.03±2.5mm. The magnetic resonance imaging was performed for all patients (G1), showing LDH and IDD in 54.3% (n=25) and 45.7% of cases (n=21), respectively.
Spine limitation was found in 15 cases.
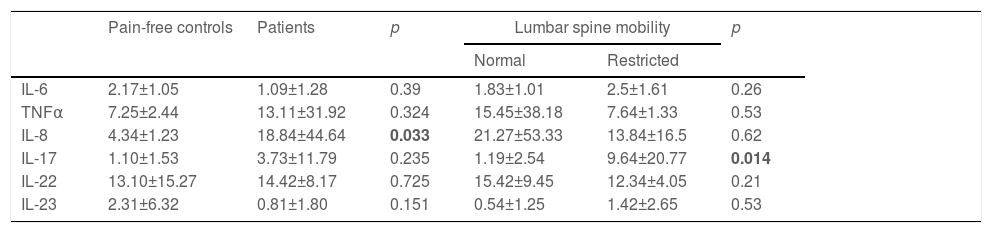
Comparison of cytokinesThe IL-8 was higher in G1 (18.84±44.64 versus 4.34±1.23pg/mL, p:0.033). There was no significant difference between the two groups regarding the other cytokines. There were no differences between patients with LDH or IDD regarding IL-8 (23.91±62.64 versus 14.95±20.64pg/mL, p:0.29) and TNFα (19.8±46.7 versus 7.2±1.3pg/mL, p:0.75).
IL-17 was higher in patients with restricted lumbar spine mobility (Table 2).
Comparison of the pro-inflammatory cytokines between the two groups.
| Pain-free controls | Patients | p | Lumbar spine mobility | p | ||
|---|---|---|---|---|---|---|
| Normal | Restricted | |||||
| IL-6 | 2.17±1.05 | 1.09±1.28 | 0.39 | 1.83±1.01 | 2.5±1.61 | 0.26 |
| TNFα | 7.25±2.44 | 13.11±31.92 | 0.324 | 15.45±38.18 | 7.64±1.33 | 0.53 |
| IL-8 | 4.34±1.23 | 18.84±44.64 | 0.033 | 21.27±53.33 | 13.84±16.5 | 0.62 |
| IL-17 | 1.10±1.53 | 3.73±11.79 | 0.235 | 1.19±2.54 | 9.64±20.77 | 0.014 |
| IL-22 | 13.10±15.27 | 14.42±8.17 | 0.725 | 15.42±9.45 | 12.34±4.05 | 0.21 |
| IL-23 | 2.31±6.32 | 0.81±1.80 | 0.151 | 0.54±1.25 | 1.42±2.65 | 0.53 |
Values were expressed as mean±SD, p: probability value, IL: interleukin, TNFα: Tumor Necrosis Factor α.
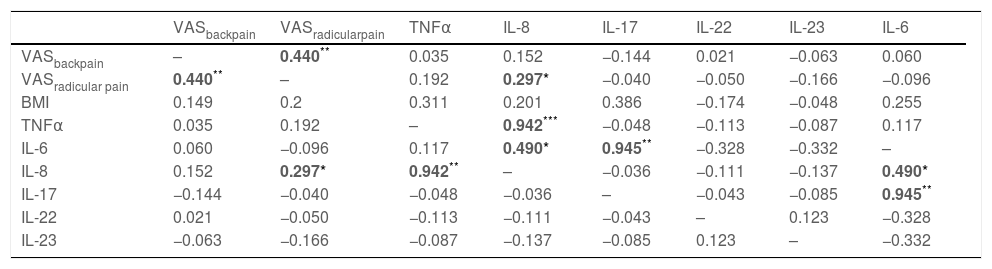
As shown in Table 3, a correlation was found between: IL-8 and TNFα (r:0.942, p<10−3), IL-8 and IL-6 (r:0.490, p: 0.011), and IL-17 and IL-6 (r:0.945, p<10−3). IL-8 levels correlated also with VASRadicular-pain (r:0.297, p:0.047).
Correlations between clinical parameter and cytokines.
| VASbackpain | VASradicularpain | TNFα | IL-8 | IL-17 | IL-22 | IL-23 | IL-6 | |
|---|---|---|---|---|---|---|---|---|
| VASbackpain | – | 0.440** | 0.035 | 0.152 | −0.144 | 0.021 | −0.063 | 0.060 |
| VASradicular pain | 0.440** | – | 0.192 | 0.297* | −0.040 | −0.050 | −0.166 | −0.096 |
| BMI | 0.149 | 0.2 | 0.311 | 0.201 | 0.386 | −0.174 | −0.048 | 0.255 |
| TNFα | 0.035 | 0.192 | – | 0.942*** | −0.048 | −0.113 | −0.087 | 0.117 |
| IL-6 | 0.060 | −0.096 | 0.117 | 0.490* | 0.945** | −0.328 | −0.332 | – |
| IL-8 | 0.152 | 0.297* | 0.942** | – | −0.036 | −0.111 | −0.137 | 0.490* |
| IL-17 | −0.144 | −0.040 | −0.048 | −0.036 | – | −0.043 | −0.085 | 0.945** |
| IL-22 | 0.021 | −0.050 | −0.113 | −0.111 | −0.043 | – | 0.123 | −0.328 |
| IL-23 | −0.063 | −0.166 | −0.087 | −0.137 | −0.085 | 0.123 | – | −0.332 |
Data are presented as the r value in Pearson's correlation test.
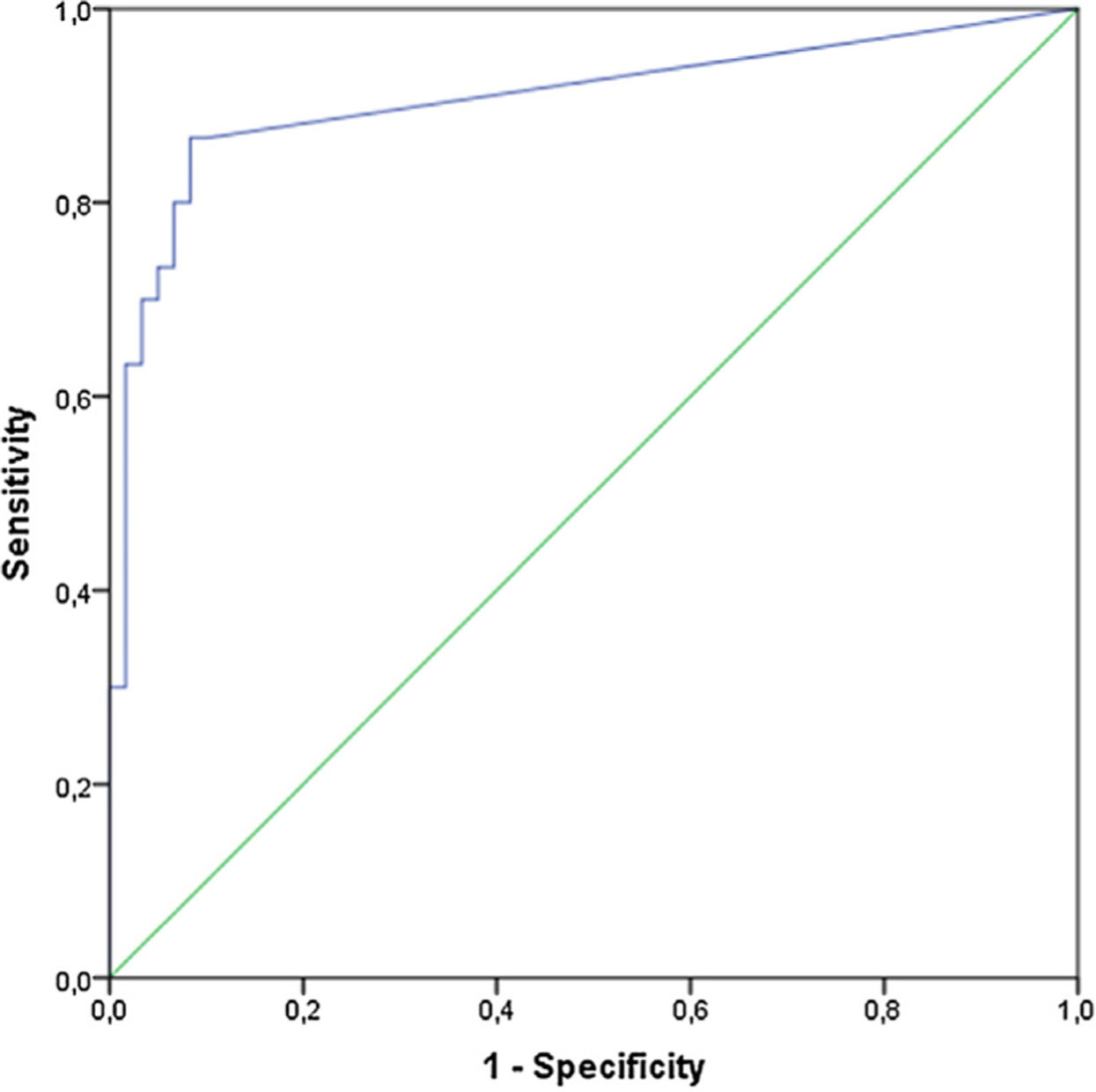
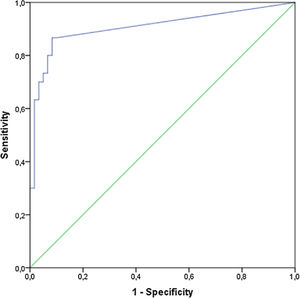
The IL-8 could differentiate patients in G1 from G0 with a cutoff of 4.5pg/mL. The area under the curve was good (0.917) (Fig. 1). The sensitivity and specificity were 91.3% and 90%, respectively.
DiscussionWe attempted to study pro-inflammatory cytokines, including IL-6, IL-8, IL-17, IL-23, IL-22, and TNFα, in patients with chronic LBP. We found that IL-8 was higher in patients with non-specific LBP, and it was able to differentiate patients with LBP from pain-free controls with a cutoff of 4.5pg/mL.
Several studies have analyzed the role of cytokines in LBP, with variable results but with a predominance of pro-inflammatory cytokines, indicating the role of inflammatory process in causing disk damage.
In a study including 127 patients with lumbar radicular pain due to LDH, the authors demonstrated that chronic pain patients had increased serum levels of IL-8 at 6 weeks and 12 months after LDH.11 These findings suggest that IL-8 may contribute to the development of chronic pain after LDH.11 Moreover, it has been proven in animal models that IL-8 was implicated in the pathophysiology of neuropathic pain.12
Likewise, Wang et al. demonstrated that IL-8 and TNFα levels were higher in patients with LBP and sciatica than healthy controls. They also found that TNFα correlated with Oswestry Disability Index (r:0.629, p:0.001)13 which is a patient reported outcomes questionnaire used to assess functional disability in back pain patients.14
This finding suggests that pro-inflammatory cytokines contribute to the generation and persistence of pain.
In a study including 155 women with acute LBP, an association was found between TNFα elevation and pain severity. TNFα was also associated with disability.15
In a longitudinal study including 120 patients with LBP matched to a healthy control group, the TNFα was positive in 57.6% LBP patients versus 12.3% of controls at the beginning of the study. The TNFα levels decreased after 10 days of multidisciplinary therapy, then remained constantly high in LBP patients. Nevertheless, no significant correlation was found between TNFα levels and pain.16
Krock et al. found that IL-8 levels in the spinal fluid were higher in patients with chronic LBP due to IDD than those with IDD without pain. The authors highlighted the effect of blocking IL-8 receptor signaling on reducing LBP behaviors and disk inflammation in a mouse model.17
Karppinen et al. assessed the serum biomarkers in chronic LBP patients with lumbar Modic change. They found that IL-8 and TNFα were lower among these patients than pain-free controls.18
Schroeder et al. showed that TNFα was higher in patients with intervertebral discs with type II Modic changes.19
These findings suggest the possible pathophysiological role of IL-8 and TNFα in LBP and radicular pain due to IDD or LDH. However, their role in LBP related to vertebral bone marrow lesions such as Modic change remains unclear and needs to be more closely assessed.19
In our study, a strong correlation was found between IL-8 and TNFα. This correlation (IL-8/TNFα r:0.35; p=0.003) was also noted in the study of Licciardone et al., highlighting the link between pro-inflammatory cytokines in non-specific LBP.20
Zhang Y et al. demonstrated that intervertebral disk cells could produce IL-1, IL-7, and IL-8.21 They suggested that these cytokines mediate discogenic pain by stimulating nerve endings in intervertebral disk and contribute to the persistence of this pain by diffusing these cytokines to nearby tissues.21
IL-8 can also promote angiogenesis and attract neutrophils in pathological sites.22
Besides, IL-8 can promote microglial activation in the lumbar disk herniation rate model.23 Chronic pain maintenance can be explained by delayed IL-8 upregulation in the spinal cord and the dorsal root ganglion.23
These findings suggest that IL-8 inhibition may be a potential therapy for chronic low back pain.
Several studies emphasize the role of IL-17 in the pathophysiology of IDD and demonstrate that IL-17 is an important regulator of inflammation in disk diseases.24,25
Besides, IL-17 may contribute to the pain genesis in patients with LBP. Cheng et al. found that VAS pain score was positively correlated with IL-17 concentration (r=0.458, p=0.007) in patients with LBP. IL-17 levels were also higher in patients with ruptured lumbar disk (12.13±3.62pg/ml) than those with non-ruptured lumbar discs (5.06±3.78pg/ml, p=0.003) and healthy controls (1.31±0.77pg/ml, p<0.001).8
In our study, IL-17 was significantly higher in patients with spine limitations.
A correlation was found between IL-6 and both IL-8 and IL-17. In a study assessing IL-6 expression in paravertebral muscle, annulus fibrosus, and nucleus pulposus biopsies, the IL-6 was found in all lumbar disk hernias biopsies but not in controls. This result suggests that the lumbar disk hernia is associated with a local inflammatory process. Nevertheless, there was no correlation between IL-6 expression and VAS scores.26
Notably, some authors highlighted that elevated pro-inflammatory cytokines levels in LBP were due to adipocytes stimulation in overweight and obese patients.27 In our study there was no correlation between cytokines levels and BMI. Pro-inflammatory cytokines like IL-6, IL-8 and TNFα correlated to BMI in subjects without LBP.28–30 To our knowledge, the correlation between pro-inflammatory cytokines was not assessed in LBP patients.
To our knowledge, this study is the first to determine an IL-8 threshold to distinguish LBP patients from healthy controls. The results of our study are encouraging. However, some limitations must be considered, notably the small number of patients with LBP.
ConclusionIL-8 seemed to be the most important pro-inflammatory cytokine in patients with disk-disease, as it was significantly higher in patients with LBP.
IL-8 correlated with VAS, highlighting the role of this cytokine in the development of chronic pain in patients with disk disease.
TNFα concentrations correlated with IL-8 and IL-17 levels were higher in patients with restricted lumbar pain, suggesting that these cytokines might play a role in the pathophysiology of disk disease. These findings could potentially be used by future studies to develop new LBP therapeutic strategies.
Authors’ contributions- -
Dr. Maroua SLOUMA: Methodology and Writing – review & editing
- -
Dr. Lobna KHARRAT: Roles/Writing – original draft
- -
Dr. Aymen TEZEGDENTI: data curation and funding acquisition
- -
Dr. Rim DHAHRI: formal analysis
- -
Dr Ezzeddine GHAZOUANI: project administration
- -
Dr. Leila METOUI: conceptualisation
- -
Dr. Imen GHARSALLAH: visualization
- -
Dr. Bassem LOUZIR: visualization and validation
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability (software application or custom code)Not applicable.
Ethics approvalThis study was approved by the ethics committee of the Hospital.
Consent to participateA consent has been signed by each patient.
FundingThe authors declare that they have no funding for the research.
Conflict of interestNone.
None.