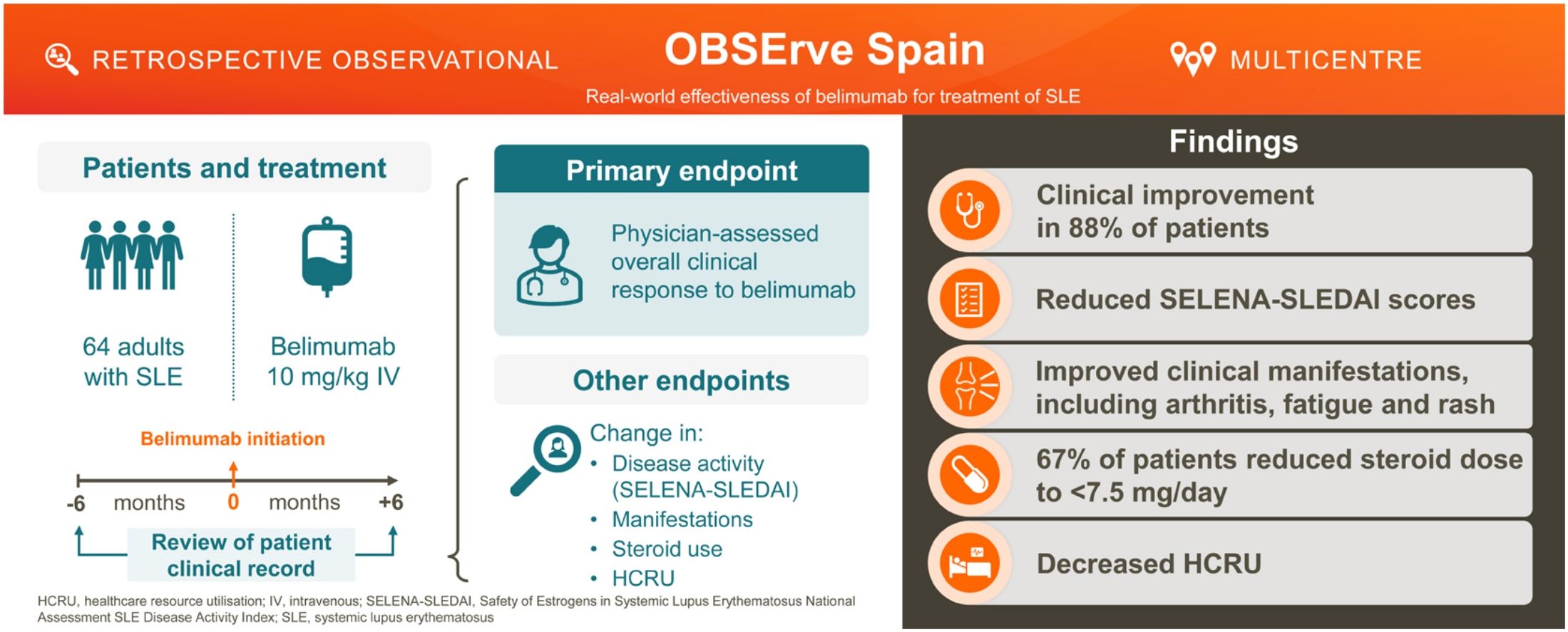
This OBSErve Spain study, a part of the international OBSErve programme, evaluated belimumab real-world use and effectiveness following 6 months of treatment in patients with active systemic lupus erythematosus (SLE) in clinical practice in Spain.
Materials and methodsIn this retrospective, observational study (GSK Study 200883), eligible patients with SLE receiving intravenous belimumab (10mg/kg) had their disease activity (physician assessed), SELENA-SLEDAI scores, corticosteroid use, and healthcare resource utilisation (HCRU), assessed after 6 months of treatment versus index (belimumab initiation) or 6 months pre-index.
ResultsOverall, 64 patients initiated belimumab, mainly due to ineffectiveness of previous treatments (78.1%) and to reduce corticosteroid use (57.8%). Following 6 months of treatment, 73.4% of patients achieved ≥20% overall clinical improvement, while only 3.1% of patients worsened. Mean (standard deviation, SD) SELENA-SLEDAI score decreased from 10.1 (6.2) at index to 4.5 (3.7) 6 months post-index. HCRU decreased from 6 months pre-index to 6 months post-index, with fewer hospitalisations (10.9% vs 4.7% patients) and ER visits (23.4% vs 9.4% patients). Mean (SD) corticosteroid dose decreased from 14.5 (12.5)mg/day at index to 6.4 (5.1)mg/day 6 months post-index.
ConclusionsPatients with SLE receiving belimumab for 6 months in real-world clinical practice in Spain experienced clinical improvements and a reduction in HCRU and corticosteroid dose.
El estudio OBSErve España, que forma parte del programa internacional OBSErve, evaluó el uso y la eficacia de belimumab en la práctica clínica real española tras seis meses de tratamiento en pacientes con lupus eritematoso sistémico (LES) activo.
Materiales y métodosEn este estudio observacional y retrospectivo (GSK Study 200883) fue evaluada la respuesta clínica, la actividad de la enfermedad (puntuación SELENA-SLEDAI), el uso de corticosteroides y los recursos sanitarios utilizados de los pacientes con LES que recibieron belimumab intravenoso (10mg/kg), al inicio y tras seis meses de tratamiento.
ResultadosEn total 64 pacientes iniciaron belimumab, principalmente por ineficacia de los tratamientos previos (78,1%) y para reducir los corticoides (57,8%). Después de seis meses de tratamiento, 73,4% de los pacientes lograron una mejoría clínica general de ≥20%, mientras que solo 3,1% de los pacientes empeoró. La puntuación media (desviación estándar, DE) de SELENA-SLEDAI disminuyó de 10,1 (6,2) a 4,5 (3,7). Los recursos sanitarios utilizados disminuyeron con menos hospitalizaciones (10,9 vs. 4,7%) y visitas a urgencias (23,4 vs. 9,4%). La dosis media (DE) de corticosteroides disminuyó de 14,5 (12,5mg/día) a 6,4 (5,1mg/día).
ConclusionesLos pacientes con LES que recibieron belimumab durante seis meses en la práctica clínica real en España experimentaron mejoras clínicas y una reducción de la dosis de corticosteroides y recursos sanitarios utilizados.
Systemic lupus erythematosus (SLE) is a complex, chronic autoimmune disorder, characterised by a broad spectrum of manifestations with a variable and unpredictable relapsing-remitting course. Despite an improvement in SLE management and prognosis, a considerable proportion of patients still experience suboptimal disease control, and increased morbidity and mortality, exacerbated by long-term SLE-related medications such as corticosteroids.1–4
Enhanced understanding of SLE pathogenesis has led to the emergence of a new drug class which targets specific immunologic pathways of the disease process.5–7 One such approach inhibits B-lymphocyte stimulator (BLyS), a key cytokine for maturation and survival of B cells, which is elevated in approximately 50% of patients with SLE.8 Belimumab, a human immunoglobulin (Ig)G1λ monoclonal antibody, blocks the binding of soluble BLyS and inhibits B-lymphocyte activity and survival.9 The efficacy and safety of belimumab has been demonstrated in five Phase 3 placebo-controlled trials in autoantibody-positive patients with SLE receiving standard therapy,10–14 and in patients with active lupus nephritis (LN).15 Whilst being mindful of cross-study comparisons, evidence from observational studies suggests belimumab efficacy in real-world settings could be greater than that reported in clinical trials.16–19
However, data from real-world clinical practice settings in Spain are limited, particularly data relating to the direct healthcare costs associated with belimumab. Understanding real-world treatment patterns and outcomes with belimumab may help foster best clinical practice, identify barriers to patient adherence/persistence, and identify patients who would benefit most from belimumab treatment. To gain insight into the effectiveness of belimumab in routine clinical practice and its impact on healthcare resource utilisation (HCRU), an observational OBSErve programme (Evaluation Of use of Belimumab in clinical practice SEttings) was initiated in the USA20 and later extended to other countries, including Spain.17 The OBSErve Spain study represents the biggest cohort of belimumab patients evaluated in routine clinical practice settings in Spain to date. Its objective was to evaluate belimumab effectiveness following 6 months of treatment, in addition to standard therapy, in patients with active SLE.
Materials and methodsStudy designOBSErve Spain (GSK Study 200883) was a retrospective, multicentre, observational chart review cohort study that collected real-world data from patient medical records between December 2013 and February 2014.
The study period comprised 12 months, subdivided into two periods of 6 months before and after the belimumab initiation date (index). Patients received belimumab at the recommended dosage of intravenous (IV) 10mg/kg every 4 weeks, after 3 induction doses 2 weeks apart, in addition to concomitant immunosuppressive medication, and remained on this dose throughout the study. Patients who discontinued belimumab within the first 6 months were also included.
The study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices (GPP). All study documents were approved by the competent national authority, the Agencia Española de Medicamentos y Productos Sanitarios, for study classification and the ethics committee of the local coordinating investigator, the Comité Ético de Investigación Clínica (CEIC) de l’Hospital Universitari Vall d’Hebrón, de Barcelona. Patient informed consent was not required.
Study populationClinical sitesPhysicians from clinical sites prescribing belimumab for ≥6 months in ≥2 patients with SLE as part of routine care qualified to recruit eligible patients.
Patient populationPhysicians enrolled adult patients (≥18 years of age) with a confirmed diagnosis of SLE and active disease receiving standard therapy, in whom belimumab therapy was initiated for the first time as part of their routine clinical care ≥6 months prior to study enrolment. Medical and treatment history for ≥6 months prior to index had to be available. Patients were excluded if they were receiving belimumab as part of a clinical trial in an interventional arm, or were currently enrolled in any SLE-related trial. Physicians included all eligible patients to avoid selection bias.
Data collectionData collection was performed using an electronic data capture system (EDC). Deidentified data from patient medical records were abstracted into electronic Case Report Forms (eCRFs). Automatic checks were implemented in the EDC to avoid missing answers and to provide valid and plausible data entries. Clinical manifestations were assessed through clinical routine evaluations. Due to the real-world, and retrospective nature of this study, clinical evaluation methods, including criteria to classify SLE, were not prospectively specified. SLE disease characteristics assessed at index included time since diagnosis, disease severity (mild, moderate and severe) and clinical manifestations, and were obtained from the medical records. Disease activity assessments were performed retrospectively (when absent) and were also included in the medical records.
Study objectivesThe primary objective was to describe the overall patterns of SLE care and outcomes among patients receiving belimumab in clinical practice in Spain. Secondary objectives were to describe the characteristics of patients receiving belimumab in clinical practice, reasons for initiation and discontinuation of belimumab, change in Safety of Estrogens in Systemic Lupus Erythematosus National Assessment SLE Disease Activity Index (SELENA-SLEDAI) score, the treatment patterns with concomitant medications (especially corticosteroids), and to describe HCRU among patients receiving belimumab.
Study endpointsThe primary efficacy endpoint was the overall clinical response, as assessed by the physicians’ judgment scale of response (Physician Global Assessment [PGA]-like scale), assessed at 6 months of belimumab treatment. PGA reflects physicians’ subjective opinion of the improvement of their patients’ SLE status, categorised as: worse, no improvement, <20% improvement, 20–49% improvement, 50–79% improvement, and ≥80% improvement, versus index.
Other endpoints (assessed at 6 months post-index) included: reasons for belimumab initiation, changes in SELENA-SLEDAI score, clinical response for specific manifestations as assessed by physicians (arthritis [including the number of tender and swollen joints], rash and fatigue) and laboratory parameters (complement C3 and C4 levels and anti-double-stranded DNA [anti-dsDNA] antibody positivity). Physician-reported changes in manifestations and laboratory parameters were reported as the proportion of patients with worse, no improvement, <20% improvement, 20–49% improvement, 50–79% improvement, and ≥80% improvement. Use of concomitant SLE medications, and corticosteroid (prednisone-equivalent) use (reduction of corticosteroid dose and switch from ≥7.5mg/day to <7.5mg/day) were included. For HCRU, proportions of patients with, and change in the number of, scheduled and unscheduled physician office visits, hospitalisations and emergency room (ER) visits were reported. Additionally, reasons for belimumab discontinuation and occurrence of adverse events (AEs) were collected.
Except for HCRU, all data were collected at index and at 6 months post-index. HCRU data were collected during the 6 months prior to index and at 6 months post-index.
Safety measuresGiven the retrospective, observation nature of the study, AEs were not systematically collected. However, deaths, hospitalisations or AEs, which may have been belimumab-related, were included in the eCRF and reported to the GSK AE reporting system. Belimumab dose modifications or discontinuations were included in the eCRF.
Statistical analysisThe analysis included all patients fulfilling eligibility criteria who had completed CRFs (full analysis set). Given the descriptive nature of the study, no sample size calculations were performed. The target sample size was 70–80 patients. For categorical data, absolute and relative frequencies of categories/items for each variable were calculated. For continuous data, mean, standard deviation (SD), minimum, median and maximum were calculated. For the analysis of concomitant corticosteroid doses, values of continuous variables were grouped into two categories: ≥7.5mg/day and <7.5mg/day, as defined previously.19
ResultsPatient populationThe full analysis set included 64 patients documented by 25 physicians at 25 clinical sites; demographic and clinical data for these patients at index are shown in Table 1. Most patients were female (n=57, 89.1%) and of Caucasian origin (n=63, 98.4%), with a mean (SD) age of 42.7 (12.1) years (range: 19–72 years). Half of the patients received their SLE diagnosis longer than 10 years ago (n=32, 50.0%); disease severity was moderate for 39 (60.9%) patients and severe for 16 (25.0%) patients at index.
Patient demographics and clinical characteristics at index, unless otherwise stated (N=64).
| Characteristic | N=64 |
|---|---|
| Age (years), mean (SD) | 42.7 (12.1) |
| Female, n (%) | 57 (89) |
| Race/ethnicity, n (%) | |
| Caucasian/white | 63 (98.4) |
| Asian | 1 (1.6) |
| Time since SLE diagnosis, mean (SD) | |
| <1 year | 1 (1.6) |
| 1–5 years | 14 (21.9) |
| 6–10 years | 17 (26.6) |
| >10 years | 32 (50.0) |
| Severity of SLE disease, n (%) | |
| Mild | 7 (10.9) |
| Moderate | 39 (60.9) |
| Severe | 16 (25.0) |
| Unknown | 2 (3.1) |
| SELENA-SLEDAI score*, mean (SD) | 10.1 (6.2) |
| High anti-dsDNA level, n (%) | 44 (68.8) |
| Low C3 ( | 36 (56.3) |
| Low C4 ( | 37 (57.8) |
| Immunosuppressant use, n (%) | |
| Azathioprine | 14 (21.9) |
| Mycophenolate Mofetil | 15 (23.4) |
| Methotrexate | 7 (10.9) |
| Tacrolimus | 1 (1.6) |
| Antimalarial use, n (%) | 39 (60.9) |
| NSAID use, n (%) | 9 (14.1) |
| Oral corticosteroid use, n (%) | 59 (92.2) |
| >7.5mg/day, n (%†) | 48 (81.4) |
| Dose (mg/day), mean (SD) | 14.5 (12.5) |
Anti-dsDSN, anti-double-stranded DNA; C, complement; NSAID, non-steroidal anti-inflammatory drug; SD, standard deviation; SELENA-SLEDAI, Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index; SLE, systemic lupus erythematosus.
Of the 64 patients, 12 (18.8%) had LN within a median time of 5.5 years (range: 0.2–17 years) prior to index. Of the patients with LN, 1 had Class V LN, 5 had Class IV LN, 5 had Class III LN and 1 had Class II LN. At the time of belimumab initiation, 5 (41.7%) patients with LN were in remission, 5 (41.7%) were experiencing persistent activity, and 2 (16.7%) were experiencing flare.
The most common reasons for initiation of belimumab were previous medication being ineffective (n=50, 78.1%), intent to decrease corticosteroid use (n=37, 57.8%), and worsening condition (n=35, 54.7%) (Table 2).
Reasons for belimumab initiation (N=64).
| n (%) | N=64 |
|---|---|
| Previous treatment regimen not effective | 50 (78.1) |
| Decrease use of corticosteroids (steroid-sparing) | 37 (57.8) |
| Patient condition worsening | 35 (54.7) |
| Previous treatment regimen not well tolerated | 13 (20.3) |
| Previous treatment regimen inconvenient | 2 (3.0) |
| Patient request | 1 (1.6) |
| Vasculitis | 1 (1.6) |
| Osteonecrosis | 1 (1.6) |
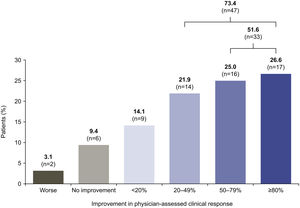
After 6 months of belimumab treatment, 47 (73.4%) patients experienced ≥20% improvement in their overall clinical condition and 33 (51.6%) patients experienced a clinical improvement of ≥50%, versus index. During this period, 6 (9.4%) patients experienced no clinical improvement and 2 (3.1%) patients worsened (Fig. 1).
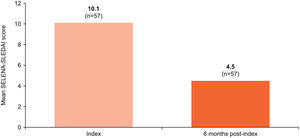
Disease activity assessmentThe mean (SD) SELENA-SLEDAI score, documented for 57 patients, showed a marked reduction, from 10.1 (6.2) at index to 4.5 (3.7) at 6 months post-index (Fig. 2).
Evaluation of clinical manifestationsClinical manifestations are shown in Table 3 and Supplementary Table 1. At belimumab initiation, the most common clinical and immunological manifestations were arthritis (56.3%), low complement levels (53.1%), increased anti-dsDNA antibody levels (48.4%), fatigue (43.8%) and inability to taper steroids (42.2%), which were rated as moderate or severe in the majority of patients. Clinical improvements were observed after 6 months of belimumab treatment. For example, of 36 patients with arthritis at index, 33 (91.7%) patients showed ≥20% improvement in their condition at 6 months post-index, of whom 25 (69.5%) showed ≥50% improvement (as assessed by physicians). For patients with arthritis in whom the 28-joint count was evaluated, a significant reduction in the mean (SD) number of tender joints (8.1 [3.6] at index [n=26] to 2.4 [3.0] at 6 months post-index [n=28]) and swollen joints (3.6 [3.6] at index [n=27] to 0.5 [1.1] at 6 months post-index [n=28]) was observed. Fatigue was present in 28 (43.8%) patients at index, and belimumab treatment resulted in an improvement of ≥20% for 22 (78.6%) of these patients. Rashes were present in 17 (26.6%) patients at index, 12 (70.6%) of whom experienced improvement of ≥20% 6 months post-index.
Physician-assessed improvements in clinical manifestations and laboratory parameters at 6 months post-index (N=64).
| Clinical manifestation and laboratory parameters*, n (%) | Index(N=64, n [%]) | Improvement from index after 6 months, n (%+) | |||||
|---|---|---|---|---|---|---|---|
| Worse | No improvement | <20% | 20–49% | 50–79% | ≥80% | ||
| Arthritis | 36 (56.3) | 0 | 1 (2.8) | 2 (5.6) | 8 (22.2) | 11 (30.6) | 14 (39.9) |
| Fatigue | 28 (43.8) | 0 | 1 (3.6) | 5 (17.9) | 5 (17.9) | 12 (42.9) | 5 (17.9) |
| Low complement(C3, C4 or CH50) | 34 (53.1) | 0 | 6 (17.7) | 6 (17.7) | 6 (17.7) | 4 (11.8) | 12 (35.3) |
| Increased anti-dsDNA antibody levels | 31 (48.4) | 2 (6.5) | 5 (16.1) | 8 (25.8) | 2 (6.5) | 7 (22.6) | 7 (22.6) |
| Inability to taper corticosteroids | 27 (42.2) | 0 | 4 (14.8) | 4 (14.8) | 6 (22.2) | 6 (22.2) | 7 (25.9) |
| Rash | 17 (26.6) | 2 (11.8) | 3 (17.7) | 0 | 3 (17.7) | 2 (11.8) | 7 (41.2) |
| Leukopenia | 15 (23.4) | 1 (6.7) | 2 (13.3) | 1 (6.7) | 6 (40.0) | 2 (13.3) | 3 (20.0) |
| Proteinuria | 12 (18.8) | 1 (8.3) | 3 (25.0) | 1 (8.3) | 3 (25.0) | 2 (16.7) | 2 (16.7) |
| Thrombocytopenia | 7 (10.9) | 0 | 2 (28.6) | 2 (28.6) | 0 | 1 (14.3) | 2 (28.6) |
| Mucosal ulcers | 7 (10.9) | 0 | 1 (14.3) | 1 (14.3) | 1 (14.3) | 2 (28.6) | 2 (28.6) |
| Alopecia | 7 (10.9) | 1 (14.3) | 0 | 1 (14.3) | 1 (14.3) | 3 (42.9) | 1 (14.3) |
Anti-dsDNA, anti-double-stranded DNA; C, complement; CH50, total haemolytic complement.
Similarly, clinical improvements were observed for most of the assessed laboratory parameters after 6 months of belimumab treatment compared with index (Table 3).
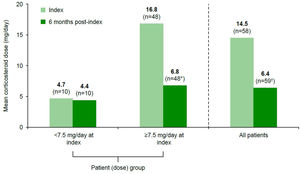
Concomitant medicationAt index, 59 (93.8%) patients were receiving oral corticosteroids, at a mean (SD) dose of 14.5 (12.5)mg/day. After 6 months of belimumab treatment, the mean (SD) corticosteroid dose decreased to 6.4 (5.1)mg/day (n=59; Fig. 3). Of 48 (75.0%) patients who received ≥7.5mg/day corticosteroids at index, 32 (66.7%) decreased their dose to <7.5mg/day and 2 (4.2%) discontinued corticosteroids, with a corresponding mean (SD) dose reduction from 16.8 (12.7)mg/day at index to 6.8 (5.6)mg/day at 6 months post-index. For patients who were receiving <7.5mg/day corticosteroids at index (n=10, 15.6%), the mean (SD) dose remained stable: 4.7 (0.9)mg/day at index and 4.4 (1.2)mg/day at 6 months post-index. One patient started corticosteroid therapy (5mg/day) during belimumab treatment.
Change in mean oral corticosteroid dose from index following 6 months of treatment with belimumab for patients with known corticosteroid use at index (n=58) and at 6 months post-index (n=59) and for patients stratified by corticosteroid dose at index (<7.5mg/day vs ≥7.5mg/day).
*Two patients discontinued corticosteroids (mean dose at index 10.0mg/day), 32 patients switched to <7.5mg/day (from mean 14.1mg/day at index to 4.5mg/day at 6 months post-index), and 14 patients remained on ≥7.5mg/day (mean dose at index 23.9mg/day, mean dose at 6 months post-index 13.2mg/day) during belimumab treatment; all these patients were included in the mean dose calculations presented here; †one patient initiated corticosteroids during belimumab treatment at a prednisone-equivalent dose of 5.0mg/day (at 6 months post-index).
The number of patients receiving other concomitant medications, including immunosuppressants, decreased after 6 months of belimumab treatment, as did most mean doses (Supplementary Table 2).
Healthcare resource utilisationA total of 63 (98.4%) patients reported ≥1 scheduled rheumatologist visit in the 6 months before index, versus 64 (100%) patients in the 6 months post-index. The mean (SD) number of scheduled visits was similar between these two time periods (6 months prior to index: 3.5 [2.2]; 6 months post-index: 3.3 [1.9]). Fewer patients required ≥1 unscheduled rheumatologist visit in the 6 months post-index (n=16, 15.0%) at a mean (SD) number of 0.3 (0.7) visit per patient, versus 6 months prior to index (n=34, 53.1%; mean [SD] number of visits: 1.0 [1.3]; Table 4). Over half of the patients (n=34, 53.1%) consulted other specialists for SLE-related reasons at index, falling to 27 (42.2%) patients in the 6 months post-index (Table 4). There was also a reduction in the number of patients requiring an SLE-related ER visit (n=15, 23.4%, reducing to n=6, 9.4%, respectively), and in the mean (SD) number of visits per patient (1.6 [1.1] visits reducing to 0.4 [0.6], respectively) from the 6 months prior to index versus 6 months post-index. In the 6 months prior to index, 7 (10.9%) patients were hospitalised (mean [SD] length of stay: 6.7 [4.3] days); 5 (7.8%) patients were hospitalised once, and 2 (3.1%) patients experienced ≥2 hospitalisations. In the 6 months post-index, 3 (4.7%) patients were hospitalised once (mean [SD] length of stay: 6.7 [3.5] days). No hospitalisations were related to belimumab use.
Summary of HCRU in the 6 months before and 6 months after initiation of belimumab treatment (N=64).
| Healthcare resources | 6 monthsprior to index | 6 monthspost-index |
|---|---|---|
| Scheduled rheumatologist visits, n (%) | 63 (98.4) | 64 (100) |
| Number of visits/patient, mean (SD) | 3.5 (2.2) | 3.3 (1.9) |
| Non-scheduled rheumatologist visits, n (%) | 34 (53.1) | 16 (15.0) |
| Number of visits/patient, mean (SD) | 1.0 (1.3) | 0.3 (0.7) |
| Other specialists visits, n (%) | 34 (53.2) | 27 (41.2) |
| ER visits, n (%) | 15 (23.4) | 6 (9.4) |
| Number of visits per patient with an ER visit, mean (SD) | 1.6 (1.1) | 0.4 (0. 6) |
| Patients with hospitalisations, n (%) | ||
| 1 hospitalisation | 5 (7.8) | 3 (4.7) |
| ≥2 hospitalisations | 2 (3.1) | 0 (0) |
| Length of stay (days) per hospitalisation, mean (SD) | 6.7 (4.3) | 6.7 (3.5) |
ER, emergency room; HCRU, healthcare resource utilisation; SD, standard deviation.
No patient required a belimumab dose modification during the study and 1 patient required a dose interruption of 1 month due to a pilonidal cyst. Belimumab was discontinued in 2 patients during the first 6 months of therapy, 1 patient due to an AE (inflammatory pelvic disease, suspected to be treatment-related and for which the sponsor did not receive evaluation) after 82 days of exposure, and 1 patient due to lack of efficacy after 154 days of exposure.
Adverse eventsTwo AEs were reported that led to belimumab discontinuation (see “Belimumab discontinuation” section for details). No deaths were reported.
DiscussionThis observational real-world cohort study conducted in Spain shows early and sustained effectiveness of belimumab during the first 6 months of treatment when added to standard SLE therapy, with a corresponding reduction in HCRU.
The most common reasons selected for initiating belimumab were lack of efficacy of other drug regimens, inability to taper corticosteroids, and persistent disease activity. After 6 months of belimumab treatment, over half of patients achieved a >50% improvement in their SLE activity, as indicated by physician-assessed clinical response, while the mean SELENA-SLEDAI score decreased substantially. These results are in line with those from other OBSErve studies,17 and several independent academic observational studies.16,18,19 The prior OBSErve studies showed a similar clinical response after 6 months of belimumab treatment, with an improvement in disease activity for 99.0% (USA20), 98.1% (Canada21), 95% (Argentina22), 92.7% (Germany23), and 83.0% (Switzerland24) of patients, compared with 87.5% of patients from Spain. When the data from all six countries were pooled, the overall clinical response was 95.3%.17 A larger proportion of Spanish patients experienced ≥80% improvement in SLE compared with other countries, which ranged from 9 to 21%, and was 13% in the pooled analysis.17 This is perhaps related to the high proportion of patients with severe disease at index in Spain (33% vs 21% in the pooled analysis).17 However, it is difficult to interpret between-country comparisons due to the subjective nature of physician assessments, and differences in patient populations between countries.
Consistent with previous studies13,18,19,21–25 and with the OBSErve pooled analysis,17 belimumab treatment was associated with a reduction in corticosteroid use. This was particularly evident for patients receiving ≥7.5mg/day of corticosteroids, of whom 66.7% switched to <7.5mg/day after 6 months of belimumab treatment, a slightly larger proportion than that in the OBSErve pooled analysis (52.6%).17 Corticosteroids were reinitiated in just 1 patient.
Fatigue is a common complaint of patients with SLE and is associated with diminished ability to function.26,27 In most cases, the cause of fatigue is unexplained; indeed, the accumulation of many factors may lead to SLE fatigue.27 Until recently, clinical trials have not routinely assessed fatigue.26 In the belimumab Phase 3 trials, Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) score improved significantly from baseline to Week 52 with belimumab treatment.10,11 In our study, a marked improvement in fatigue after 6 months of belimumab treatment was observed. However, these results should be interpreted cautiously, as it was a subjective evaluation by the treating physician and no objective or patient-reported measurements like FACIT-F score were performed.
The HCRU data from Spain were consistent with those for the USA, showing an overall reduction in HCRU after 6 months of belimumab treatment.20 Both studies recorded a reduction in unscheduled rheumatologist and ER visits. OBSErve Spain also documented a decrease in hospitalisations, similar to that shown in OBSErve US study. Furthermore, belimumab was generally well tolerated, with a low number of AEs and discontinuations in this study, similar to the OBSErve pooled analysis.17
This study has some limitations, including a lack of a control group and a strict protocol. Furthermore, there were no formal statistical analyses and only descriptive data are presented. Patient populations and treatment regimens may have varied across sites and may not be representative of non-participating sites. Clinical response was evaluated based on physicians’ judgement, which is subjective, and the applied scale might be conceived differently by another cohort of physicians. Data were collected and validated electronically, and verification was not performed. It should also be noted that the observation period ended in 2014, and thus patient profile data may not be fully aligned to the current clinical care and to the management and classification of SLE published in 2019.28–30 Finally, the short observation period may miss treatment-related AEs or flares that may occur after prolonged treatment. A longer observational period may be required to fully capture longitudinal treatment effect. Indeed, in the OBSErve Argentina study, which evaluated belimumab treatment up to 24 months post-index, 26% of patients experienced a clinical improvement of ≥80% after 6 months of belimumab treatment, rising to 80% of patients after 24 months of treatment.22 Therefore, future studies should include a longer observation period and formal statistical analyses, in which further improvements may be detected and quantified.
ConclusionIn this study evaluating belimumab treatment in real-world clinical practice settings in Spain, a notable improvement in SLE disease activity, based on clinical and serological manifestations and improvement in SELENA-SLEDAI score, was observed among patients with SLE receiving belimumab for 6 months, which was accompanied by a decrease in HCRU. In addition, in this short treatment period, a significant reduction of concomitant corticosteroids was observed. The low number of belimumab discontinuations due to AEs indicates that, in general, belimumab was well tolerated. These findings support the continued use of belimumab for the management of SLE in Spain across different SLE manifestations. However, further observational studies, particularly with subcutaneous formulations of belimumab, are required to fully elucidate the effectiveness potential of this biological agent on patient outcomes, adherence and impact on healthcare systems.
Conflicts of interestJ. Cortés-Hernández received consulting fees from GSK. C. Marras received consulting fees from GSK. J.L. Andreu received consulting fees from Eli Lilly, Sanofi, UCB and AstraZeneca. J. Calvo received consulting fees from GSK, Eli Lilly and MSD. A.M. Garcia received consulting fees from AbbVie, GSK and BMS. E. Diez declares to have no conflict of interest. F.J. Hidalgo and A. Perna were full-time employees of GSK at the time of the study and held stock options in GSK. C. Coronell was employed by GSK at the time of the study. J. Ordi received consulting fees from GSK.
OBSErve Spain study (GSK Study 200883) was funded by GSK, and conducted by Kantar Health GmbH, Munich, Germany (including support for study design and statistical analyses) and Kantar Health Spain (study sites management). Medical writing support was provided by Nicholas Thomas, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
FundingThis study (GSK Study 200883) was funded by GSK and conducted by Kantar Health GmbH, Munich, Germany (including support for study design and statistical analyses) and Kantar Health Spain (study sites management). The sponsor (GSK) contributed to the design, collection, analysis and interpretation of the data, and the decision to submit the manuscript for publication, and supported the authors in development of the manuscript. Medical writing support was provided by Nicholas Thomas, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
This study (GSK Study 200883) was funded by GSK. The authors would like to thank the participating patients and their families, clinicians, and the following OBSErve Spain study investigators: Alegre Sancho J.J. (Valencia), Andreu Sanchez J.L. (Madrid), Blanco Alonso R. (Cantabria), Calvo Alen J. (Cantabria), Castañeda Sanz S. (Madrid), Cortes-Hernández J. (Barcelona), De Ramón Garrido E. (Málaga), Díez Alvarez E. (León), Fernández Espartero C. (Madrid), Galindo Izquierdo M. (Madrid), García Aparicio A.M. (Toledo), González Fernández J.A. (Alicante), Graña Gil J. (La Coruña), Hernández Beriain J.A. (Las Palmas), Hernández Rodriguez I. (Pontevedra), Marras Fernández-Cid C. (Murcia), Mirón Trigueros P. (Almería), Narváez García F.J. (Barcelona), Navarro Blasco F. (Alicante), Ordi Ros J. (Barcelona), Paulino Huertas M. (Ciudad Real), Pozuelo Lopez M.J. (Valencia), Rodriguez Escalera C. (Melilla), Rodriguez Heredia J.M. (Madrid), Rosas Gómez de Salazar J.C. (Alicante), Saiz Cuenca E. (Murcia). Moreover, the authors would like to acknowledge the role of Volker Koscielny, former GSK Immunoinflammation Global Medical Affairs Leader, in the conception and design of OBSErve Spain study. Medical writing support of the first manuscript draft was provided by Claudia Kanitscheider, medical writer from Kantar Health GmbH (Germany), and, of the subsequent manuscript, by Nicholas Thomas, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.