There is an increasing interest in the study of non-criteria antiphospholipid antibodies (aPL) including antibodies targeting domain 1 of the B2 glycoprotein 1 (anti-D1 B2GP1) and antibodies anti phosphatidylserine/ prothrombin (PS/PT).
ObjectivesOur aim was to analyze a panel of conventional and non-criteria aPL in a cohort of patients with systemic lupus erythematosus (SLE) and primary antiphospholipid syndrome (APS), to describe if there are differences in aPL titers among groups, to evaluate clinical associations including risk of recurrent events of novel aPL.
MethodsObservational study that evaluated at baseline antibodies against anti-D1 B2GP1 and anti PS/PT. Anti-D1 B2GP1 antibodies were tested using a chemiluminescent immunoassay. IgG and IgM anti PS/PT, aCL and anti B2GP1 by ELISA techniques. Therefore, patients were followed in order to identify new thrombotic events.
Results133 patients with SLE and 23 with primary APS patients were included. Main APS manifestations were DVT (27%), obstetric morbidity (22%) and arterial thrombosis (10.1%). IgM anti PS/PT antibodies levels were (20.6 - 127) vs 21.9 (11.2 - 39.2) U/ml, p<0.001 in primary APS vs SLE with APS, respectively. Anti-D1 B2GP1, IgG and IgM anti PS/PT were associated with thrombotic and non-thrombotic manifestations. During follow-up, IgG B2GP1 were related with a significant cumulative risk of thrombosis.
ConclusionsWe found significant differences in serum titers of non-criteria aPL among patients with primary APS vs SLE with APS. Whether non-criteria aPL antibodies titers are useful to differentiate patients with primary and secondary APS requires further analysis in other populations.
Hay un interés creciente en el estudio de los anticuerpos antifosfolípidos (aPL) no criterio, incluyendo anticuerpos contra el dominio 1 de la B2 glicoproteína 1 (anti-D1 B2GP1) y anticuerpos antifosfatidilserina/protrombina (PS/PT).
ObjetivosNuestro objetivo fue analizar un panel de aPL convencionales y no criterio en una cohorte de pacientes con lupus eritematoso sistémico (LES) y síndrome antifosfolípido primario (SAF), para describir si hay diferencias en los títulos de aPL entre los grupos, y evaluar asociaciones clínicas incluyendo el riesgo de eventos recurrentes con aPL novedosos.
MetodologíaEstudio observacional que evaluó los anticuerpos anti-D1 B2GP1 y anti-PS/PT de manera basal. Los anticuerpos anti-D1 B2GP1 se evaluaron a través de inmunoanálisis por quimioluminiscencia. Los anticuerpos anti-PS/PT, anticardiolipinas (aCL) y anti-B2GP1 fueron evaluados por técnicas de ELISA. Finalmente, los pacientes fueron seguidos en el tiempo para identificar nuevos eventos trombóticos.
ResultadosSe incluyeron 133 pacientes con LES y 23 pacientes con SAF primario. Las principales manifestaciones de SAF fueron TVP (27%), morbilidad obstétrica (22%) y trombosis arterial (10,1%). Los títulos de anticuerpos anti-PS/PT IgM fueron 46,5 (20,6-127) vs. 21,9 (11,2-39,2) U/ml, p<0,001, en pacientes con SAF primario vs. LES con SAF secundario, respectivamente. Los anti-D1 B2GP1, anti-PS/PT IgG e IgM se asociaron con manifestaciones trombóticas y no trombóticas. Durante el seguimiento, los anticuerpos IgG B2GP1 se relacionaron con un riesgo acumulativo significativo de trombosis.
ConclusionesSe encontraron diferencias estadísticamente significativas en títulos séricos de aPL no criterio en pacientes con SAF primario vs. pacientes con LES y SAF secundario. Si los títulos de aPL no criterio son útiles para diferenciar entre SAF primario y SAF secundario, se requieren más análisis en otras poblaciones para poder confirmar si los títulos de aPL no criterio.
Antiphospholipid antibodies (aPL) are a heterogeneous family of antibodies that are directed against phospholipids and phospholipid-binding proteins. Currently. conventional serological markers for antiphospholipid syndrome (APLS) include lupus anticoagulant (LA) and anticardiolipin antibodies (aCL) using solid phase assays.1 There is growing interest in novel aPLs. especially antibodies against B2 glycoprotein 1 domain 1 (anti-D1 B2GP1) and anti-phosphatidylserine/prothrombin (PS/PT) antibodies. among others. Novel aPLs may provide additional information in risk stratification. and identify patients with persistently negative conventional aPL determinations. also known as seronegative APLS patients.2
PFS is defined as primary or associated with an underlying autoimmune disease. Primary APLS occurs in patients in whom there is no clinical or laboratory evidence of another condition. On the other hand. associated or secondary APLS may be associated with other diseases. most commonly systemic lupus erythematosus (SLE).1
Assessment of the aPL profile has diagnostic implications and aids in risk stratification. Triple aPL positivity has been associated with high risk of a first and recurrent thrombotic event.3,4 and is an independent risk factor associated with gestational complications such as lower live birth rate. higher rate of intrauterine growth restriction (IUGR). placental abruption. among others.5
Anti-D1 B2GP1 and anti-PS/PT IgG/IgM antibodies were significantly higher in patients with APLS than those without APLS.6 An Italian study of patients with primary APLS7 found that several clinical and serological features were associated with high anti-PS/PT antibody titres. IgG and IgM anti-PS/PT antibody titres were significantly higher in patients with thrombosis and obstetric morbidity than in those with thrombosis or obstetric morbidity alone. Another study of patients with APLS who were seeking conception found that anti-PS/PT antibodies were significantly associated with late pregnancy complications. IUGR and pre-eclampsia. In addition. anti-PS/PT IgG antibody titres had a statistically significant inverse correlation with neonatal birth weight.8 A systematic review found that routine measurement of anti-PS/PT antibodies (IgG and IgM isotypes) could be useful to establish thrombotic risk in patients with previous thrombosis or SLE.9
Anti-D1 B2GP1 antibodies have been associated with thrombotic events. including venous and arterial thrombosis.10,11 and have been found in high titres in patients with multiple aPL positivity.12,13
To date. the significance of aPL titres. especially novel aPLs. in patients with primary APLS is unclear. The aim of this study was to analyse a panel of both conventional and novel aPLs in patients with SLE and primary APLS. to describe the differences in aPL titres in both groups. and to evaluate the clinical associations of novel aPLs. including the risk of recurrent events.
MethodsStudy populationThis observational study included clinical evaluation and serological determination of baseline autoantibodies in patients with SLE and primary APLS. followed by prospective follow-up. The diagnosis of SLE was made according to the American College of Rheumatology (ACR) classification criteria (1982/199714 and the APLS according to the Sidney criteria.15 Primary APLS was defined according to the criteria proposed by Piette et al.16 Patients with active cancer or prothrombotic disease other than APLS were excluded.
Patients ≥ 18 years were recruited from the Department of Rheumatology. Internal Medicine and the Anticoagulation Clinic. Hospital San Vicente Fundación. Medellín. Colombia: a tertiary referral centre. between March 2015 and March 2017. with follow-up until March 2019.
A systematic review of medical records was performed for demographic variables. aPL-related criteria. and non-criteria manifestations. Arterial and venous thrombotic events were recorded. Arterial events included: myocardial infarction (including vascular procedures for myocardial infarction). angina pectoris and cerebrovascular attacks. Venous events recorded were deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE). Only the first incident event was recorded. Obstetric morbidity was recorded according to the Sidney criteria for APLS.15
Written informed consent to participate was obtained from the patients. The present study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committee of the Hospital San Vicente Fundación. within the project “Biomarkers in systemic lupus erythematosus” (Act number 10-2015).
Patients were followed for new thrombotic events or APLS-related events until the occurrence of an event or the last visit. All anti-thrombotic therapies used during follow-up, including vitamin K antagonists and direct oral anticoagulants, were recorded.
Determination of antibodiesAnti-D1 B2GP1 antibodies were determined by QUANTA Flash® (Beta2)GP1-Domain 1 chemiluminescence immunoassay (Inova Diagnostics. San Diego. CA. USA). Anti-PS/PT IgG and IgM antibodies were assayed by QUANTA Lite ELISA (Inova Diagnostics. San Diego. CA. USA). The aCL (IgG and IgM) and anti-B2GP1 (IgG and IgM) were measured simultaneously by ELISA techniques (QUANTA Lite®. Inova Diagnostics). To detect LA. internationally accepted recommendations were used. using diluted Russell's viper venom and activated partial thromboplastin time (aPTT) as screening methods.17 The aforementioned antibodies were determined only at the time of patient inclusion.
Statistical analysisStatistical analyses were performed in SPSS® software (IBM SPSS® version 23.0. Inc. Chicago. IL. USA) and GraphPad Prism version 7.0 (GraphPad Software. San Diego. CA. USA). Serum titres of anti-D1 B2GP1 and anti-PS/PT were compared between patients with SLE and primary APLS. and between patients with SLE and APLS vs. patients with primary APLS. In addition. serum antibody titres were compared between patients with SLE without thrombosis. SLE with thrombosis and primary APLS. Chi-square test and Mann-Whitney or Kruskall-Wallis U-test were used to compare dichotomous and continuous variables. respectively. In addition. for continuous variables with non-normal distribution. the Wilcoxon rank test was used. Spearman's correlation test was used to look for correlations between aPL (aCL. anti-PS/PT and anti-B2GP1). Correlation size was interpreted as very high, high, moderate, low, and negligible according to values between .9-1.00, .7-.89, .5-.69, .3-.49, 0-.29, respectively. Finally. Kaplan-Meier time-to-event analysis was used to determine risk factors for thrombotic events during follow-up.
ResultsPatient characteristicsFrom an initial cohort of 173 patients. complete clinical data and serum samples were available for 156 patients (133 with SLE and 23 with primary APLS).
The demographic. clinical and serological baseline characteristics of all patients. those with SLE and those with primary APLS are shown in table 1. The mean age was 33.9±12.8 years. and 86% were female: 77% were of mixed race and 22% were Afro-Latin American. The main criteria manifestations of APLS in the total cohort were DVT (27%). obstetric morbidity (22.9%) and arterial thrombosis (10.1%). The main non-criteria manifestations were thrombocytopenia (31.6%). valvular heart disease (21.5%) and previous seizures (12.1%). Serologically. LA was positive in 55.9% of patients. aCL IgG in 30%. aCL IgM in 17%. B2GP1 IgG in 24% and B2GP1 IgM in 14.5% of patients. New aPL positivity was: anti-PS/PT IgG (33%). PS/PT IgM (40%) and anti-D1B2GP1 (19.9%). One fifth of patients were double positive for LA+any aCL and 8.3% were triple positive for conventional aPL (LA+any aCL+any B2GP1). Twenty-seven percent of SLE patients had a history of thrombosis. Because we included patients who met the Sidney criteria for APLS, none had single positivity for new aPLs with negative conventional aPLs.
Clinical and serological characteristics.
| Total cohort | Primary APLS | SLE without APLS | SLE with APLS | |
|---|---|---|---|---|
| Clinical characteristics | N=156 | N=23 | N=88 | N=45 |
| Sex (female). % | 86 | 82 | 84 | 93 |
| Current age (years±SD) | 33.9±12.8 | 41.1±16.4 | 30.4±10.4 | 36.6±13.1 |
| Criteria manifestations | ||||
| Previous thrombosis (arterial and/or venous). % | 35.7 | 86.4 | 0 | 70.0 |
| Deep vein thrombosis. % | 27 | 54.5 | 0 | 59.0 |
| Pulmonary thromboembolism. % | 9.8 | 21.0 | 0 | 23.0 |
| Arterial thrombosis. % | 10.1 | 40.0 | 0 | 14.0 |
| Obstetric morbidity. % | 22.9 | 54.5 | 0 | 51.0 |
| Non Criteria manifestations | ||||
| Valvulopathy. % | 21.5 | 28.6 | 0 | 8.3 |
| Thrombocytopenia. % | 31.6 | 42.8 | 23.0 | 41.0 |
| Convulsions. % | 12.1 | 4.8 | 5.0 | 25.0 |
| Livedo reticularis. % | 6.7 | 19.0 | 6.0 | 2.3 |
| Serological manifestations | ||||
| Standard aPLs | ||||
| Lupus anticoagulant positive. % | 55.9 | 60.9 | 34.0 | 65.0 |
| IgG aCL positive. % | 30.0 | 60.0 | 26.0 | 30.0 |
| IgM aCL positive. % | 17.0 | 40.0 | 10.0 | 12.0 |
| IgG B2GP1 positive. % | 24.0 | 55.0 | 15.0 | 27.0 |
| IgM B2GP1 positive. % | 14.5 | 30.0 | 6.7 | 12.0 |
| Double positive (LA+any aCL) | 19.9 | 43.5 | 6.3 | 18.0 |
| Triple positive (LA+any aCL+any B2GP1) | 8.3 | 0 | 2.8 | 7.5 |
| New aPLs | ||||
| Anti-D1 B2GP1 positive. % | 19.9 | 43.0 | 9.6 | 26.0 |
| IgG anti-PS/PT positive. % | 33.0 | 60.0 | 16.0 | 51.0 |
| IgM anti-PS/PT positive. % | 40.0 | 75.0 | 36.0 | 42.0 |
aPL: Antiphospholipid antibodies; APLS: Antiphospholipid syndrome; SD: Standard Deviation; SLE: Systemic Lupus Erythematosus.
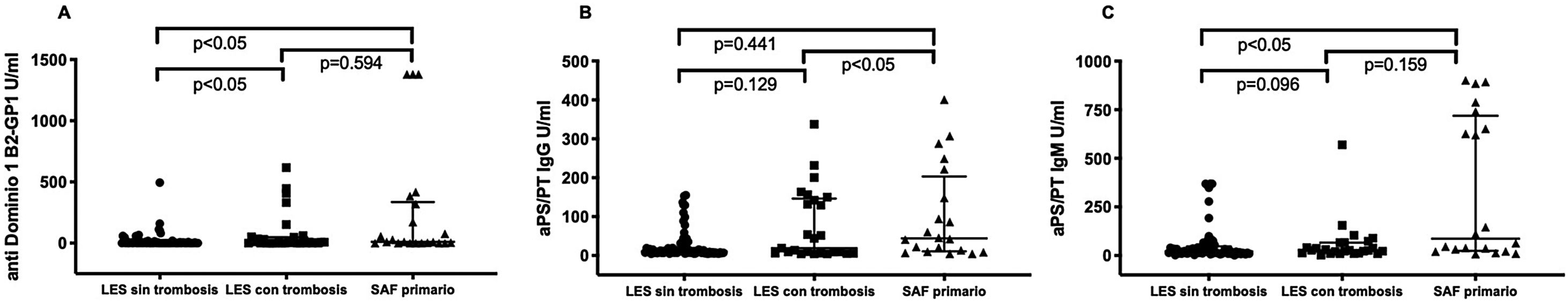
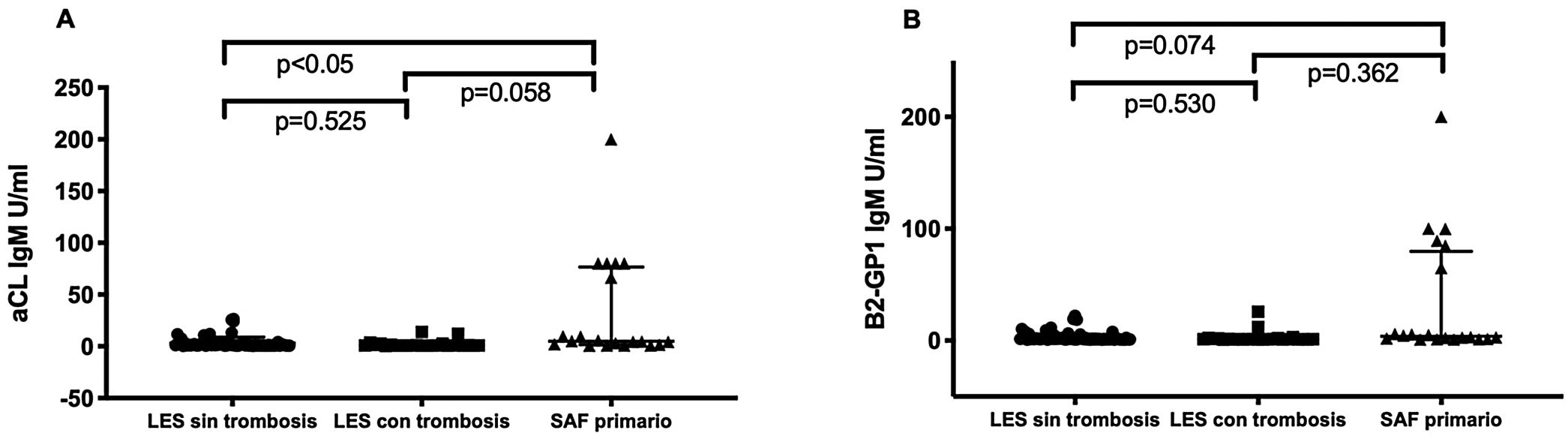
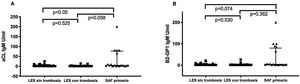
Patients with primary APLS had significantly higher titres of certain new aPL than patients with SLE without APLS. Patients with primary APLS had significantly higher titres of anti-PS/PT IgG and IgM than patients with SLE and APLS (46.5 [20.6-127] vs. 21.9 [11.2-39.2] median interquartile range [IQR]) U/ml. p<.001 in patients with primary APLS vs. SLE and APLS respectively. No statistically significant differences were observed in anti-D1 B2GP1 titres. 0 (0-8) vs. 5 (0-53) median (RIQ) U/ml. p=.163 between both groups (fig. 1). Patients with primary APLS and thrombosis had significantly higher titres of aCL IgM and B2GP1 IgM than patients with SLE and APLS and patients with SLE without APLS (fig. 2).
Anti-PS/PT antibody titres (IgG and IgM) were significantly higher in patients with triple positivity for classical aPL. No difference in anti-D1 B2GP1 titres was observed in patients with or without triple positivity (data not shown). and no significant difference in new aPL titres was observed in patients with DVT alone vs. patients with arterial thrombosis alone.
Anti-D1 B2GP1 was strongly associated with previous thrombosis (both arterial and venous), odds ratio (OR): 4.90. 95% confidence interval (CI): 2.0-11.7; DVT (OR: 5.0. 95% CI: 2.1-11.9) and thrombocytopenia (OR: 4.3. 95% CI: 1.8-10.3). Anti-PS/PT IgG was strongly associated with previous thrombosis, DVT, thrombocytopenia and obstetric morbidity while anti-PS/PT IgM was associated with previous thrombosis and thrombocytopenia (table 2).
Clinical performance of anti-D1 B2GP1 and anti-PS/PT IgG and IgM antibody assays in patients with features of APLS (both primary APLS and SLE with APLS).
| Anti-D1 B2GP1 positive (%) | Anti-D1 B2GP1 negative (%) | OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Thrombosis (any arterial or venous) | 65.5 | 34.5 | 4.9 | 2.0-11.7 | <.001 |
| DVT | 55.2 | 44.8 | 5.0 | 2.1-11.9 | <.001 |
| Thrombocytopenia | 58.6 | 41.4 | 4.3 | 1.8-10.3 | <.001 |
| IgG anti-PS/PT positive (%) | IgG anti-PS/PT negative (%) | OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Thrombosis (any arterial or venous) | 54.8 | 22.9 | 4.0 | 1.7-9.3 | <.001 |
| DVT | 60.6 | 24.1 | 5.2 | 1.3-20.8 | <.001 |
| Thrombocytopenia | 26.5 | 4.9 | 7.0 | 1.9-24.7 | <.001 |
| Obstetric morbidity | 64.7 | 22 | 6.4 | 2.0-20.0 | <.001 |
| IgM anti-PS/PT positive (%) | IgM anti-PS/PT negative (%) | OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Thrombosis (any arterial or venous) | 52.8 | 32.1 | 2.3 | 1.0-5.2 | .034 |
| Thrombocytopenia | 26.5 | 4.9 | 7.0 | 1.9-24.7 | <.001 |
95% CI: 95% Confidence Interval; APLS: Primary Antiphospholipid Syndrome; DVT: Deep vein thrombosis; OR: Odds Ratio; SLE: Systemic Lupus Erythematosus.
A positive correlation was observed between anti-D1 B2GP1 and anti-PS/PT IgG (r=.651; p=.0001). B2GP1 IgG (r=.604; p=.0001) and aCL IgG (r=.645; p=.0001). Previously known moderate-strong correlations were observed between conventional aPL (table 3).
Correlation between new and conventional aPLs.
| Anti-D1 B2GP1 | IgG anti-PS/PT | IgM anti-PS/PT | IgG B2GP1 | IgM B2GP1 | IgG aCL | IgM aCL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho | P value | Rho | P value | Rho | P value | Rho | P value | Rho | P value | Rho | P value | Rho | P value | |
| Anti-D1 B2GP1 | 1 | — | .651 | .0001 | .548 | .0001 | .604 | .0001 | .346 | .0001 | .645 | .0001 | .445 | .0001 |
| IgG IgM anti-PS/PT | .651 | .0001 | 1 | — | .552 | .0001 | .554 | .0001 | .324 | .0001 | .657 | .0001 | .334 | .0001 |
| IgM IgM anti-PS/PT | .548 | .0001 | .552 | .0001 | 1 | — | .504 | .0001 | .412 | .0001 | .592 | .0001 | .523 | .0001 |
| IgG B2GP1 | .604 | .0001 | .554 | .0001 | .504 | .0001 | 1 | — | .0001 | .822 | .0001 | .592 | .0001 | |
| IgM B2GP1 | .346 | .0001 | .324 | .0001 | .412 | .0001 | .496 | .0001 | 1 | — | .456 | .0001 | .822 | .0001 |
| IgG aCL | .675 | .0001 | .657 | .0001 | .592 | .0001 | .822 | .0001 | .456 | .0001 | 1 | — | .542 | .0001 |
| IgM aCL | .445 | .0001 | .334 | .0001 | .523 | .0001 | .592 | .0001 | .823 | .0001 | .542 | .0001 | 1 | — |
aPL: Antiphospholipid antibodies.
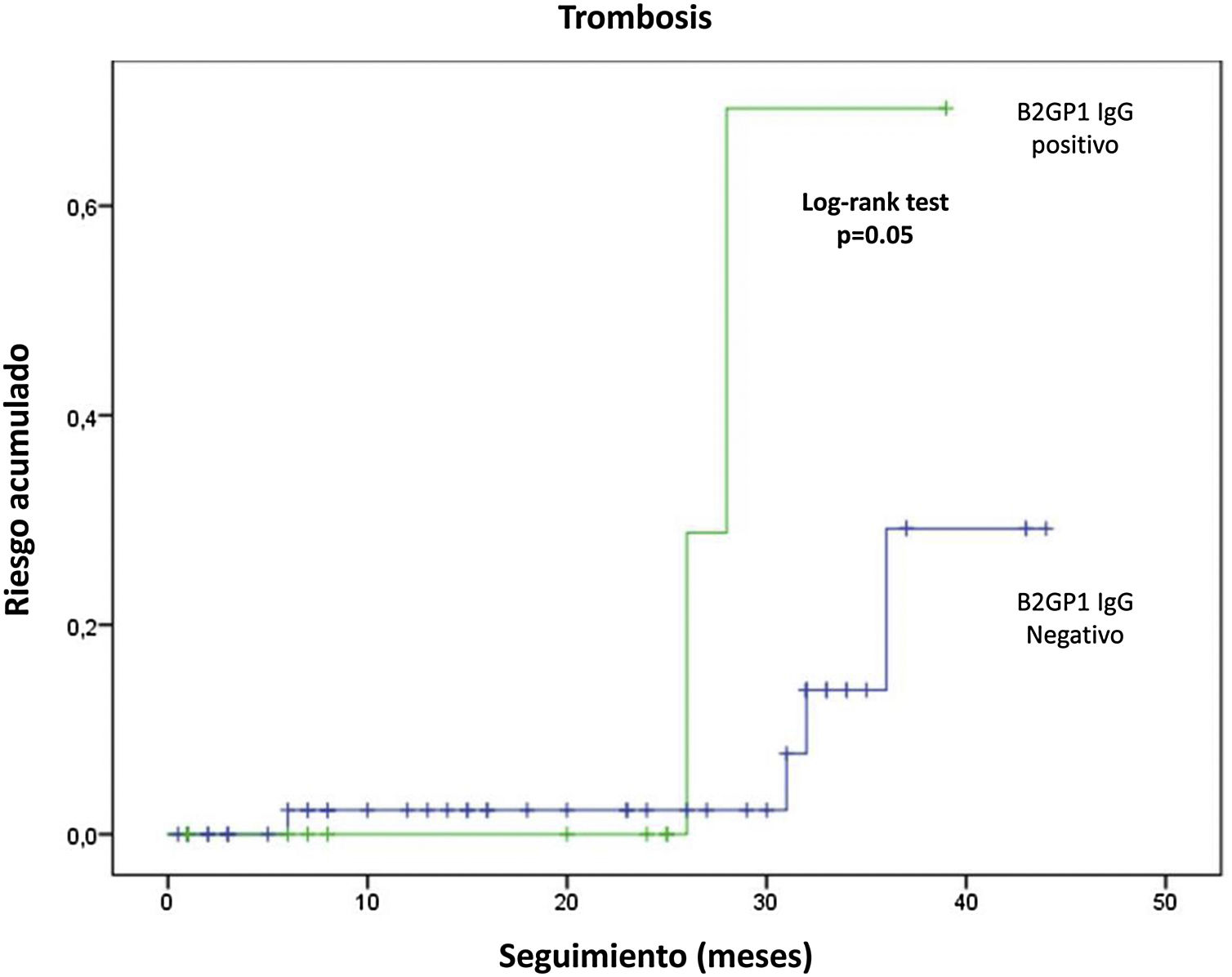
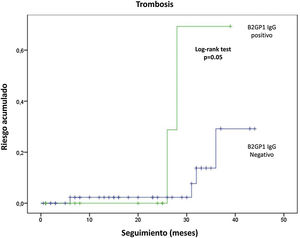
Follow-up was available in 91 patients. Mean follow-up time was 20.6±13.8 months (range: 1-46 months). Eight of 91 patients (8.8%) had new thrombotic events (6 DVT and 2 arterial thromboses). 5 of which occurred despite anticoagulant therapy (4 with vitamin K antagonists and one with dabigatran). Six episodes of thrombocytopenia and two new fetal losses were observed. Overall. LA was associated with recurrent thrombocytopenia (12.9 vs. 0%; p=.049) and anti-B2GP1 IgG antibodies with recurrent thrombosis (28 vs. 7%; p=.022) and arterial thrombosis (6.7 vs. 0%; p=.05). In survival analysis. anti-B2GP1 antibodies were significantly related to the cumulative risk of thrombosis (fig. 3). No association was observed between aPL non-criterion or triple positivity and recurrence. No clinically relevant bleeding (major or minor) was observed.
DiscussionOur results show significant differences in anti-PS/PT titres between patients with primary APLS and patients with SLE and APLS. In addition, strong associations were found between new aPL with criterion (thrombosis and obstetric morbidity) and non-criterion manifestations such as thrombocytopenia.
Information on the differential role of new aPLs is limited. In a Chinese study of patients with primary APLS (n=101). secondary APLS (n=140) and controls, including patients without thrombosis (n=161) and healthy controls (n=39), serum titres of anti-PS/PT IgG and IgM were significantly higher in patients with primary APLS and secondary APLS compared to patients with thrombosis without APLS, with obstetric morbidity without APLS, with SLE without thrombosis and healthy controls. However. anti-PS/PT IgG or IgM levels were not significantly different between patients with primary APLS and secondary APLS.18
Non-criteria aPLs, including anti-B2GP1 IgA, anti-D1 B2GP1 and anti-PS/PT have been proposed as useful markers in patients with seronegative APLS (patients with clinical manifestations of APLS although with negative conventional aPL results).19
Although the new aPLs are not usually measured in daily clinical practice, some studies have suggested that they are useful as adjunctive tests to identify patients with APLS seronegative for conventional aPLs.20,21 However, since we did not include any cases of seronegative APLS, this possible additional diagnostic value was not evaluated in the present study.
Primary APLS is a well-recognised entity, progressing to SLE or other autoimmune diseases in only .6%-11% of patients. In 2005, in a multicentre cohort study including 128 patients with primary APLS, we reported that about 8% of patients met SLE criteria after a median follow-up of 9 years.22 In a European cohort of 1,000 patients with APLS, after a 10-year follow-up, only 3/531 patients (.6%) initially diagnosed as primary APLS were reclassified as SLE.23 In another European multicentre study of 115 patients with APLS, only 13 (11%) were reported to develop frank autoimmune disease, 7 patients developed SLE, 2 Sjögren's syndrome and 4 undifferentiated connective tissue disease after a follow-up of 18 years.24 A recent study of 100 French patients with primary APLS found that none developed SLE after a median follow-up of up to 12 years.25
Multiple disease markers are not exclusive to their most representative diseases, but high titres are often a hallmark of each disease. For example, patients with ANCA-associated vasculitis often have higher ANCA titres than patients with other ANCA-positive inflammatory diseases, including patients with inflammatory bowel disease or SLE.26
Despite a short follow-up in our study, about 9% of patients had new thrombotic episodes, in some cases in patients on oral anticoagulation. In particular, patients positive for anti-B2GP1 IgG antibodies had an increased risk of recurrence. A recent study reported a higher rate of thrombotic events in those patients with positive anti-PS/PT IgG27 and Abu-Zeinag et al.28 reported that 18% of patients developed a thrombotic event after 48 months of follow-up, especially those with an underlying autoimmune disease. However, as in our study, they did not document a relationship with LA positivity or triple positivity.
There is no consensus on monitoring aPL titres during follow-up and on the impact of aPL levels. Recently, Khawaja et al.29 described that the presence of aCL and, to a lesser extent. LA, can be negative after thrombotic events in SLE patients. Nuri et al.30 found a significant reduction in aPL titres in patients with primary APLS exposed to hydroxychloroquine (HCQ) compared to those without HCQ, and a potential reduction in the incidence of recurrence of arterial thrombosis in patients with primary APLS treated with HCQ.
Our study has some limitations:
- -
First, we only measured aPL at the time of study inclusion and therefore cannot determine whether anti-D1 B2GP1 and anti-PS/PT titres changed over time.
- -
Second, a relatively low number of patients with primary APLS were included compared to those with SLE plus APLS, but in the same ratio (1:4) as usually seen in clinical practice between primary APLS and SLE-associated APLS. Despite such a relatively low number of cases, we were able to report that serum anti-PS/PT titres were twice as high in patients with primary APLS as in those with SLE and APLS. However, some of these differences were not statistically significant, although with a greater tendency for elevated anti-D1 B2GP1 titres in patients with primary APLS. While the statistically significant finding was reduced to anti-PS/PT and aCL antibodies, there is a numerical difference in anti-D1 B2GP1 antibody titres in favour of patients with primary APLS. However, the finding may not be significant due to the small sample size. This is in agreement with our findings of high titres of other non-criteria antibodies.
- -
Third, we found a high prevalence for AL positivity compared to other cohorts. This may be due, in part, to the recruitment site of many patients with primary APLS who came from the anticoagulation clinic after a positive confirmatory LA test.
- -
Fourth, we were unable to identify patients with seronegative APLS because all patients were classified as APLS according to the criteria of at least one classical aPL at recruitment. The study period was relatively short, but we were nevertheless able to identify new thrombotic episodes. Finally, most of our patients were of mixed race and. therefore our findings are not necessarily generalisable to other populations.
In conclusion, to our knowledge, this is the first study to describe differences in serum titres of new aPL in patients with primary APLS or SLE with APLS. This may generate hypotheses on the differential role of new aPL in patients with both primary APLS and secondary APLS related to systemic autoimmune diseases. Whether anti-D1 B2GP1 and/or anti-PS/PT titres are useful in discriminating between patients with primary or secondary APLS requires further analysis.
Conflict of interestsThe authors have no conflict of interests to declare.
Anti-D1 B2GP1 antibodies were kindly provided by INOVA/Werfen Colombia. The authors thank Munther A. Khamashta and David Buss for their critical review of the content of this manuscript. We also thank Sandra M. Osorno and Angela Loaiza (Dinámica IPS. Colombia) for support with sample handling and analysis of aCL and anti-B2GP1 antibodies.