MiDAS study assessed the percentage of psoriatic arthritis (PsA) patients treated in routine clinical practice who achieved control of disease activity according to Disease Activity in Psoriatic Arthritis (DAPSA) and Minimal Disease Activity (MDA).
MethodsObservational, non-interventional, cross-sectional, multicenter study conducted under conditions of routine clinical practice in 36 centers with outpatient rheumatology clinics in Spanish public hospitals. Patients included were adults (≥18 years) with ≥6 months PsA diagnosis according to classification for PsA (CASPAR) criteria and undergoing treatment ≥3 months. The main variable evaluated was the percentage of patients under remission and low disease activity, assessed through DAPSA and MDA.
Results313 patients with PsA were included: 54.3% male; with mean age of 54.1±12.2 years and mean disease duration of 10.5±9.0 years. Mean C-reactive protein (CRP) serum levels were 4.9±7.3mg/L. At the study visit, 58.5% of patients were in monotherapy (17.6% biological and 40.9% non-biological) and 41.2% were receiving biological and non-biological therapy.
59.4% of patients showed low disease activity (DAPSA≤14) and 19.8% were on remission (DAPSA≤4). Moreover, 51.4% of the patients reached an MDA status (≥5 MDA).
ConclusionsAround 40% of PsA patients presented uncontrolled disease, highlighting the need to improve the management of these patients in clinical practice.
El estudio MiDAS evaluó el porcentaje de pacientes con artritis psoriásica (APs) tratados en práctica clínica habitual que lograron el control de la actividad de la enfermedad de acuerdo con Disease Activity in Psoriatic Arthritis (DAPSA) y Minimal Disease Activity (MDA).
MétodosEstudio observacional, no intervencionista, transversal, multicéntrico, realizado en condiciones de práctica clínica habitual en 36 centros con consultas externas de reumatología de hospitales públicos españoles. Los pacientes incluidos eran adultos (≥18 años) con ≥6 meses de diagnóstico de APs según los criterios de clasificación de la APs (CASPAR) y en tratamiento durante ≥3 meses. La variable principal evaluada fue el porcentaje de pacientes en remisión y baja actividad de la enfermedad, evaluados mediante DAPSA y MDA.
ResultadosSe incluyeron 313 pacientes con APs: 54,3% varones; con una edad media de 54,1±12,2 años y una duración media de la enfermedad de 10,5±9,0 años. Los niveles séricos medios de proteína C reactiva fueron de 4,9±7,3mg/L. En la visita del estudio, el 58,5% de los pacientes estaban siendo tratados con monoterapia (17,6% biológicos y 40,9% no biológicos) y el 41,2% recibían terapia biológica y no biológica.
El 59,4% de los pacientes mostró baja actividad de la enfermedad (DAPSA≤14) y el 19,8% estaban en remisión (DAPSA≤4). Además, el 51,4% de los pacientes alcanzó un estado de MDA (≥5 MDA).
ConclusionesAlrededor del 40% de los pacientes con APs presentaban enfermedad no controlada, destacando la necesidad de mejorar el manejo de estos pacientes en la práctica clínica.
Psoriatic arthritis (PsA) is a chronic inflammatory musculoskeletal disease, generally associated with psoriasis. Its different forms and manifestations make its management complex and may require the collaboration of different specialists, mainly rheumatologists and dermatologists.1,2
Overall, the annual incidence of PsA ranges from 3.6 to 7.2 per 100,000 person years, and the prevalence in the general population is around 1–2 per 1000.1 In patients with psoriasis the incidence of PsA around 2.73 and the reported prevalence ranges between 6% and 41%.1 In Spain, EPISER study estimated the PsA prevalence in 0.58%, higher than the previously published in other European countries, and higher than the PsA prevalence estimated when our study was designed (around 0.25%).4
The Spanish Society of Rheumatology (SER) recommendations establish that the therapeutic objective in PsA is to control inflammation and preserve the functional capacity of patients, achieving clinical remission or minimum/low disease activity according to the different validated indexes.5 Although SER does not specify any specific index, Disease Activity in PsA (DAPSA) and Minimal Disease Activity (MDA) constitute the two indexes recommended by international experts to define therapeutic objectives in PsA.6,7
Currently, the treatment of PsA includes different biological and non-biological drugs, depending, among others, on the PsA phenotype and the disease activity status.2,5 In addition to the well-known conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), the development of new targeted biological DMARDs (bDMARDs) have expanded the range of therapeutic options for PsA patients to better cover the different domains of the disease and, thereby, to improve treatment response.8 Moreover to use treat-to-target approach in the management of PsA can improve outcomes and it is supported by international recommendations,9–12 although the implementation of this approach in routine clinical practice is controversial and apparently low.11
BIOBADASER registry assessed bDMARDs discontinuation and its causes in clinical practice in Spain. Although the availability of valid data from this type source, it does not substitute the value of a Real-World Evidence (RWE) studies. In this sense, there is a lack of evidence in real practice setting about several practice issues such as long-term outcomes, the patterns of drug prescription, the adherence to treatment guidelines and finally the therapy cost-effectiveness.13 The aim of this study was to assess the management of PsA patients in routine clinical practice in Spain and to determine the percentage of treated patients who achieve the objective of low disease activity according to DAPSA and MDA scores.
Materials and methodsStudy designMiDAS is an observational, non-interventional, descriptive, cross-sectional, retrospective, and multicenter study, carried out in 36 Spanish public hospitals between December 10th, 2018 and August 14th, 2019. The study included two different cohorts of outpatients: 313 patients diagnosed with ankylosing spondylitis and 313 patients diagnosed with PsA. Here, we present the PsA patients’ outcomes, including its main objective: to evaluate the percentage of PsA patients treated in routine clinical practice who presented, disease activity under reasonable control according to the national and European recommendations.5,14
Main data were collected in a single routine clinical visit, in which the patient also completed the study questionnaires and answered different questions about their perception of the disease and pain control (cross-sectional data). Additional data, including previous therapeutic regimes, laboratory, and radiographic evaluation, were retrospectively recruited from the patients’ medical records (Fig. 1). The study variables were recorded by an ad-hoc electronic Case Report Form (eCRF) specifically designed for this study.
Inclusion criteria comprised adult patients aged ≥18 years, with medically confirmed diagnosis of PsA according to classification for PsA (CASPAR) criteria for ≥6 months and undergoing treatment ≥3 months before the inclusion who provided informed consent. Patients participating in any other treatment study or who suffered serious concomitant diseases that may influence the evaluation of the PsA disease (neoplasia, uncontrolled psychiatric diseases, etc.) were excluded.
Each center included patients from its database who met all the selection criteria in the study, in a randomized way. In those centers where random selection using the database was not possible, inclusion was carried out consecutively during the scheduled visits at the Rheumatology unit. With this objective, each researcher randomly invited all PsA patients who came to her office and met all the selection criteria to participate in the study, up to a maximum of 8 patients.
To assess the percentage of PsA patients who had controlled disease activity5,14 the main study variable was the DAPSA index, and the key secondary variable was MDA. A DAPSA score >4 and ≤14 is defined as low disease activity and DAPSA≤4 is defined as remission. MiDAS study considered that DAPSA≤14 reflected a controlled disease activity (Supplementary material, Table 1).15
The percentage of patients with PsA with minimal disease activity or remission was also evaluated by the fulfillment of MDA criteria and very low disease activity (VLDA) according to the MDA definition and a DAPSA score ≤4. It is considered to have achieved an MDA when 5 of the following 7 criteria are met: number of painful joints [0–68]≤1; number of swollen joints [0–66]≤1; Psoriasis Area and Severity Index (PASI)≤1 or body surface area (BSA)≤3, patient [0–100] (in the present study we used the BSA variable); pain score on a visual analog scale (VAS) [0–100]≤15; global disease activity assessed by the patient using a VAS [0–100]≤20; Health Assessment Questionnaire-Disability Index (HAQ-DI) [0–3]≤5; painful enthesis points [0–29]≤1 according to Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) and Spondyloarthritis Research Consortium of Canada (SPARCC) indexes. When patients achieve 7 of the 7 criteria, they are considered as VLDA (Supplementary material, Table 1).15,16
The study was performed according to guidelines on observational post-authorization studies for medicinal products for human use specified in Order SAS/3470/2009 of The Spanish Agency of Medicines and Medical Devices (AEMPS). The study was conducted according to Good Clinical Practice (International Conference of Harmonization) guidelines, the Declaration of Helsinki and following the local regulation, including privacy laws, at the time of the initiation of the study. Protocol, informed consent form and other information for patients were approved by the Ethical and Clinical Research Committee of the 12 de Octubre hospital, with the ethics approval number 18/437.
Statistical methodsWhen the study was designed, the PsA prevalence was estimated to be around 0.25%,4 and internal data5 estimated that 50% of PsA patients on treatment would have a controlled disease. Considering statistical criteria, the result of the primary endpoint (% of patients with DAPSA≤14) was expected to be close to 50%, a conservative value which allowed the maximum sample size. A minimum of 267 valid patients was considered necessary to estimate the primary endpoint, with a precision of ±6% in its 95% confidence interval, and to allow 15% of invalid patients, a predicted number of 315 patients should be recruited to achieve this number of valid patients.
Continuous variables were described by mean, standard deviation (SD), median, minimum and maximum and, depending on the distribution of the analyzed variable, quartiles. The descriptive analysis was based on the valid data per parameter, excluding patients with missing values. Data were analyzed with Statistical Analysis System (SAS) Enterprise Guide 7.15, considering a significance level of 0.05 for all the analyses performed.
ResultsA total of 342 PsA patients were included in the study and 313 (91.5%) of them were evaluable. 29 (8.5%) patients were excluded from the analysis (due to non-compliance of the inclusion and/or exclusion criteria and/or incomplete study questionnaires) (Fig. 2).
Baseline characteristicsMean (SD) age of evaluable patients was 54.1 (12.2) years, 54.3% were male and 42.4% overweight. Mean (SD) disease duration was 10.5 (9.0) years and the mean time (SD) between the onset of symptoms and diagnosis was 3.2 (5.8) years (Table 1).
Baseline demographic and clinical characteristics of the evaluable population.
| PsA patients (N=313) | |
|---|---|
| Sociodemographic data | |
| Age (years), mean (SD) | 54.1 (12.2) |
| Sex (male), n (%) | 170 (54.3%) |
| BMI (kg/m2), mean (SD) | 27.8 (4.6) |
| Low weight (BMI<18.35), n (%) | – |
| Normal weight (18.5≤BMI<25), n (%) | 85 (28.8%) |
| Overweight (25≤BMI≤30), n (%) | 125 (42.4%) |
| Obesity (BMI>30), n (%) | 85 (28.8%) |
| Missing, n | 18 |
| Smoking habit | |
| Smoker, n (%) | 51 (16.3%) |
| Packets/year (smokers), mean (SD) | 14.3 (10.4) |
| Former smoker (without smoking>6 months), n (%) | 79 (25.2%) |
| Non-smoker, n (%) | 165 (52.7%) |
| Not available, n | 18 (5.8%) |
| Employment situation | |
| Unemployed, n (%) | 20 (6.4%) |
| Employee (excluding sick leave due to study illness), n (%) | 149 (47.6%) |
| On sick leave (due to study illness), n (%) | 18 (5.8%) |
| Pensioner, n (%) | 57 (18.2%) |
| Other (e.g., students, housework, etc.), n (%) | 30 (9.6%) |
| Not available, n (%) | 39 (12.5%) |
| Clinical data | |
| Family history of PsA, n (%) | 34 (10.9%) |
| Time of evolution of PsA, years, mean (SD) | 10.5 (9.0) |
| Time from onset of PsA symptoms, years, mean (SD) | 13.7 (10.4) |
| Time from onset of PsA symptoms to diagnosis, years, mean (SD) | 3.2 (5.8) |
| Family history of psoriasis, n (%) | 118 (37.7%) |
| Time of evolution of psoriasis, years, mean (SD) | 18.8 (14.1) |
| HLA-B*27 | |
| Positive, n (%) | 34 (10.9%) |
| Negative, n (%) | 160 (51.3%) |
| Not available, n (%) | 119 (38.1%) |
| CRP, mg/L, mean (SD) | 4.9 (7.3) |
| Presence of concomitant pathology, n (%) | 193 (61.7%) |
| Diabetes mellitus, n (%) | 32 (10.2%) |
| Dyslipidaemia, n (%) | 97 (31.0%) |
| Cardiovascular disease, n (%) | 17 (5.4%) |
| Kidney disease, n (%) | 3 (1.0%) |
| Hepatic steatosis, n (%) | 13 (4.2%) |
| Hypertension, n (%) | 92 (29.4%) |
| Osteoporosis, n (%) | 19 (6.1%) |
| Others, n (%)a | 120 (38.3%) |
BMI, body mass index; CRP, C-reactive protein; HLA-B*27, human leukocyte antigen-B*27; PsA, psoriatic arthritis; SD, standard deviation.
At baseline, 193 (61.7%) patients had comorbidities, being the most frequent dyslipidemia (31.0%), hypertension (29.4%) and diabetes mellitus (10.2%). Data on human leukocyte antigen-B*27 (HLA-B*27) status was available in 61.9% of the patients, being positive in 10.9% of the total population and, regarding the most recent C-reactive protein (CRP) determination, mean (SD) was 4.9 (7.3)mg/L (Table 1).
58.8% of patients were receiving bDMARDs alone or in combination. Of these, 17.6% in monotherapy.
Regarding the prescription of biological drugs, the most used mechanism of action was the inhibition of the tumor necrosis factor (TNFi, 71.7%), being adalimumab (28.6%) and etanercept (22.3%) the most used bDMARDs, followed by the inhibition of the interleukin 17 (15.8%) where secukinumab was the most used (86.2%). As csDMARDs, methotrexate (73.1%) was the most used (Table 2).
Treatments at the initial visit.
| PsA patients (N=313) | |
|---|---|
| Biological treatment,n(%) | 184 (58.8%) |
| Adalimumab, n (%)a | 53 (16.9%) |
| Etanercept, n (%)a | 41 (13.1%) |
| Infliximab, n (%)a | 9 (2.9%) |
| Golimumab, n (%)a | 18 (5.8%) |
| Certolizumab pegol, n (%)a | 11 (3.5%) |
| Ustekinumab, n (%)a | 23 (7.3%) |
| Secukinumab, n (%)a | 25 (8.0%) |
| Ixekizumab, n (%)a | 4 (1.3%) |
| Non-biological treatment,n(%) | 257 (82.1%) |
| DMARDs, n (%) | 207 (66.1%) |
| Apremilast | 14 (4.5%) |
| Metotrexate | 153 (48.9%) |
| Sulfasalazine | 15 (4.8%) |
| Leflunomide | 38 (12.1%) |
| Cyclosporin A | 2 (0.6%) |
| NSAIDs, n (%) | 121 (38.7%) |
DMARD, disease modifying antirheumatic drug; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis.
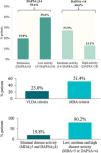
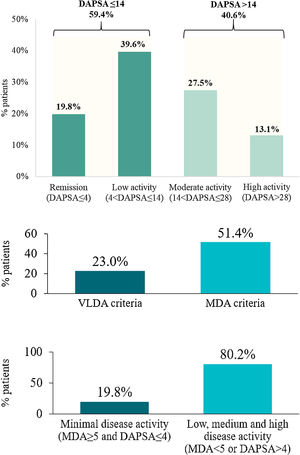
A disease-controlled status (DAPSA≤14) was noted on 59.4% of the PsA patients. This percentage was higher when patients were treated with bDMARDs (64.3%) compared with those who received non-biological treatment (52.3%). In contrast, according to MDA, a slightly lower percentage of patients treated with bDMARDs (50.3%) showed inactive disease compared with those treated with non-biologics (53.1%). The mean (SD) value from DAPSA was 15.0 (13.8), corresponding to a moderate disease activity on average. Furthermore, 19.8% of patients (23.8% treated with bDMARDs and 14.1% with non-biologicals) were in remission (DAPSA≤4) and 40.6% (35.7% with bDMARDs and 47.7% with non-biologicals) in moderate or high disease activity (DAPSA>14) (Fig. 3a).
Disease control in PsA patients (N=313). Disease activity according to DAPSA. Disease activity according to MDA and VLDA. Remission according to MDA and DAPSA. DAPSA, disease activity in psoriatic arthritis; MDA, minimal disease activity; PsA, psoriatic arthritis; VLDA, very low disease activity.
On the other hand, 51.4% of PsA patients achieved an MDA status and 44.7% of them (23.0% of the total) met VLDA criteria (Fig. 3b). The percentage of patients achieving an MDA status was slightly higher when patients received non-biological treatment (53.1%) compared with those who received bDMARDs (50.3%). However, percentages were inverted for patients achieving VLDA criteria (20.3% for patients with non-biological versus 24.9% with bDMARDs).
When combining DAPSA and MDA to evaluate disease control as an exploratory endpoint, 19.8% of the patients showed inactive disease (MDA≥5 and DAPSA≤4 criteria) while 80.2% showed active disease (MDA<5 and DAPSA>4) (Fig. 3c).
DiscussionMiDAS study was designed to evaluate the number of PsA patients treated in routine clinical practice in Spain who presented disease control. According to the primary endpoint, 59.4% of patients included achieved disease control (DAPSA≤14), with only 19.8% of the total patients in remission (DAPSA≤4). 51.4% of the PsA patients reached an MDA and 23.0% met the VLDA criteria. When combining DAPSA and MDA, 19.8% of the patients showed inactive disease (MDA≥5 and DAPSA≤4 criteria).
The therapeutic goal in patients with PsA is to achieve clinical remission or minimum/low disease activity according to the different validated indexes.5 In the present study, the indexes used to evaluate PsA activity were DAPSA and MDA, which have been broadly used in post hoc analyses, real-world studies and registries17,18 and are also included in the recent treat-to-target recommendations.19 DAPSA has cut-offs for different activity states, being a more specific measure of joint-related disease activity than other variables used in PsA pivotal clinical trials (as DAS-28 or the American College of Rheumatology [ACR] 20/50/70 response).14 Also, better DAPSA responses have been correlated with a lower probability of radiographic progression in patients with PsA.20 However, while DAPSA assesses only peripheral arthritis, the MDA criteria assesses relevant clinical outcomes across several domains of PsA and both measures are being increasingly used in clinical and research practice.12,14 In this sense, MDA is the only measure currently tested in a treat-to-target strategy trial.11
Our study revealed that, according to DAPSA, 19.8% of the PsA patients were in remission and only 59.4% in low disease activity (controlled disease). Recent studies have disclosed similar percentages of remission (DAPSA≤4 for 21.1%21 and 22.9%22 of the patients). Regarding to patients treated with bDMARDs, our study showed 23.8% of them in remission, a lower percentage than the founded in Lubrano et al., which showed 36% of patients achieving DAPSA remission after 12 months of TNFi therapy, even though in this study a different cut-off for DAPSA remission was used (DAPSA≤3.3).23
In the present study, 51.4% of the patients reached an MDA status and 23.0% fulfilled the VLDA criteria. These percentages of patients are similar and higher, respectively, to the ones reported in previous studies in our setting, where 58.6% of the PsA patients treated with different therapeutic combinations achieved an MDA24 and 11.5% a VLDA state.22 In prospective studies with biological treatments, better results have been observed versus the 50.3% of patients treated with bDMARDs of our study who achieved an MDA status, as well, Haddad et al. and Perrotta et al. showed that up to 64% and 61.3% of patients treated with TNFi drugs, respectively, achieved MDA after 12 months.25,26
Real-world studies, such as the present one, are useful to evaluate the results obtained with the available treatments in heterogeneous samples of patients (with variability in sociodemographic characteristics, time of evolution of the disease or symptoms, among others), far from the homogeneity required by clinical trials to assess new therapeutic alternatives.27 Although clinical trials continue being essential to evaluate the efficacy and safety of drugs or health interventions, real-world studies provide supplementary data of effectiveness representing the current clinical practice.28
In our study, each participating physician recruited patients who consecutively attend to the physician's office and 61.7% of them showed comorbidities at baseline, maybe because they attend physician's office more often for its symptomatology and were more probably recruited. In this sense, baseline percentage of comorbidities is higher than others founded in previous studies, which were near to 50%.29,30
Our results are consistent with those reported in previous studies, however MiDAS study also entails limitations due to its design. In this sense, the retrospective, cross-sectional design of the study allows a description of the patient's current health status but does not detect changes over time depending on the evolution of the disease. However, the details obtained in the present study suggest the need to improve the management of the PsA patients. Also, the randomized inclusion process from database patients could entail some limitations due to the randomized way as such, which may be different depending on the center. Nevertheless, and taking into account the experience of the participating researchers and centers, this bias is assumed to be minimal, especially considering that this fact has no impact on the main objective of the study. The characteristics of the study may be representative of outpatients from tertiary reference hospitals (e.g. selection bias of high complexity patients), so the extrapolation of all PsA population may be due with caution. In this regard, these patients might have a higher prevalence of comorbidities and symptomatology and results related to burden of disease may be overestimated.
ConclusionsThis study highlights the difficulties to achieve a well-controlled disease in PsA patients in routine clinical practice, showing that a 40.6% of the studied population is inadequately controlled despite being under treatment. Also, the use of standardized and validated indices such as DAPSA and MDA is a fundamental element that allows the continuous improvement of healthcare activity with the final goal of improving patient health outcomes. The existing data on PsA patients did not provide enough information on the control of disease activity. The MiDAS study has revealed the situation of PsA patients in terms of control of their disease within the context of routine clinical practice in outpatient rheumatology clinics.
FundingThis study was funding by Novartis Farmacéutica, S.A.
Conflict of interestJordi Gratacós reports personal fees from Novartis, during the conduct of the study; grants and personal fees from MSD, grants and personal fees from Pfizer, grants and personal fees from AbbVie, grants and personal fees from Janssen, grants and personal fees from Lilly, grants and personal fees from Amgen, outside the submitted work.
José L. Pablos reports personal fees from Pfizer, personal fees from Novartis, personal fees from Roche, personal fees from AbbVie, personal fees from Sanofi, personal fees from Bristol, personal fees from Gilead, personal fees from Galapagos, during the conduct of the study.
Eugenio de Miguel reports personal fees from Novartis, during the conduct of the study; grants and personal fees from Novartis, grants and personal fees from AbbVie, grants and personal fees from Pfizer, personal fees from MSD, personal fees from BMS, personal fees from Janssen, grants and personal fees from Roche, personal fees from UCB, personal fees from Lilly, personal fees from Galapagos, outside the submitted work.
Xavier Juanola reports and Personal Fees: Monies paid to you for services rendered, generally honoraria, royalties, or fees for consulting, lectures, speakers bureaus, expert testimony, employment, or other affiliations.
Cristina Fernández-Carballido reports personal fees from Novartis, during the conduct of the study; personal fees from AbbVie, personal fees from Celgene, personal fees from Janssen, personal fees from Lilly, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Roche, personal fees from UCB, outside the submitted work.
Rafael Ariza has nothing to disclose.
Pau Terradas-Montana is a Novartis employee.
Carlos Sastré reports personal fees from Novartis Farmacéutica S.A, outside the submitted work.
Cristina Sanabra reports personal fees from Novartis Farmacéutica S.A, outside the submitted work.
The authors would like to thank all investigators who participated in the MIDAS study (Supplementary material) and IQVIA and Carmen Barrull and Elena Torres for providing medical editorial assistance with this manuscript.