Nerve conduction studies (NCS) have been considered as the gold standard in carpal tunnel syndrome (CTS) diagnosis, despite correlation between clinical symptomatology and NCS severity has shown to be poor. In fact, clinical symptoms precede NCS changes in months or years. Few papers have been published about the clinical response to treatment of clinically typical CTS, but with normal NCS (NNCS).
ObjectiveTo compare the clinical response to local corticosteroid injections (LCI) in clinically typical CTS, with NNCS and abnormal NCS (ANCS).
MethodWe included patients older than 18, with typical CTS symptoms (ongoing daily nocturnal pain/paresthesias in hand, at least during 3 months). Follow-up was done at 3, 6 and 12 months. Primary outcome was the visual analog scale for pain (p-VAS), comparing NNCS CTS wrists with ANCS CTS wrists. Statistic signification was established by the Student's t test, Mann–Whitney's “U”, χ2 test and Yates’ correction.
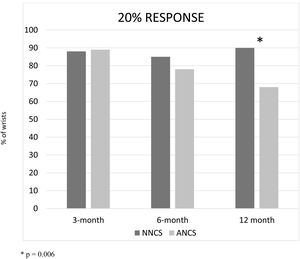
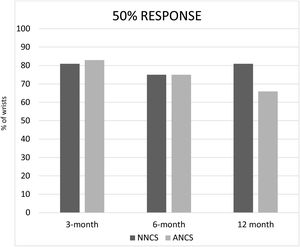
ResultsWe included 44 wrists in the NNCS group, and 83 in the ANCS group. There was no statistical significance between data in both groups, except in the 12-month follow-up, where the NNCS group achieved better results than the ANCS group in the 20% response (p=0.006). There was a trend toward a better 50% response in the 12-month follow-up.
ConclusionsOur data suggest that LCI are similarly effective in both CTS with NNCS and ANCS. Nonetheless, there is a mild better effect in NNCS than in ANCS at 12-month follow-up.
Los estudios de conducción nerviosa (NCS) se consideran el patrón oro diagnóstico del síndrome del túnel carpiano (CTS), aunque la correlación entre síntomas clínicos y gravedad de NCS es escasa. De hecho, los síntomas preceden a los cambios en NCS en meses o años. Hay pocos estudios publicados sobre la respuesta al tratamiento del CTS con sintomatología típica, pero NCS normales (NNCS).
ObjetivoComparar la respuesta clínica a infiltraciones locales de corticoides en CTS con sintomatología típica, con NNCS vs. NCS anormales (ANCS).
MétodoIncluimos pacientes mayores de 18 años, con síntomas típicos de CTS (dolor y/o parestesias continuas, nocturnas, en la mano, un mínimo 3 meses seguidos). El seguimiento se realizó a los 3, 6 y 12 meses. La medida de resultado primaria fue por la escala analógica visual para el dolor, comparando los CTS con NNCS y los CTS con ANCS. Establecimos la significación estadística mediante la «t» de Student, «U» de Mann-Whitney, el test de χ2 y la corrección de Yates.
ResultadosIncluimos 44 muñecas en el grupo de NNCS y 83 en el grupo de ANCS. No hubo diferencias estadísticamente significativas entre ambos grupos, excepto en el seguimiento a 12 meses, en donde el grupo NNCS obtuvo mejores resultados que el grupo ANCS en la respuesta al 20% (p=0,006). Hubo una tendencia similar en la mejoría de la respuesta al 50%, en el seguimiento a 12 meses.
ConclusionesNuestros datos sugieren que las infiltraciones locales de corticoides son de similar eficacia en ambos grupos de CTS, los de NNCS y los ANCS. No obstante, hay una discreta mejoría en el grupo NNCS sobre el ANCS en el seguimiento a 12 meses.
Carpal tunnel syndrome (CTS) is the clinical condition resulting from entrapment of the median nerve where it passes in the wrist through the carpal tunnel, just below the transverse carpal ligament. It is by far the most common entrapment neuropathy, and its most common etiology is idiopathic.1,2 CTS has a constellation of symptoms (pain, tingling, burning or numbness, in the distribution of the median nerve) and signs (Tinel, Phalen, Durkan, etc.). Classically, CTS diagnosis is based on clinical history, physical examination and nerve conduction studies (NCS).1
The pathogenesis of CTS is a compression of the median nerve in the carpal tunnel, but this is a very complex process.3 There are too many possible factors implicated, and some more are probably unknown yet. The main known factors include: female sex, hypothyroidism, diabetes mellitus, pregnancy, rheumatoid arthritis, higher BMI, size of carpal tunnel, repetitive injuries in wrist, trigger digit, deposit of different molecules in the transverse carpal ligament (amyloid, glycosaminoglycans), etc.4,5
Clinical symptoms may precede NCS changes by months or years. Discrepant findings have been published and correlation between clinical symptoms and NCS has been shown to be very poor,6 while other studies showed the contrary.7
This compressive mechanism likely takes a variable period in each patient. Therefore, it looks quite plausible that the median nerve compression starts being symptomatic at earlier stages, likely when the NCS is still unable to show abnormalities. At least in some (or perhaps many) patients with short symptoms onset (few weeks or months), it may be that it is a matter of time before their NNCS could turn into ANCS. Furthermore, there is also the possibility that in some patients this process will never turn into ANCS. There may be other variables that are not well understood; it has been published that in some patients with CTS and ANCS, after a period and without any active treatment, their symptoms vanish and, at least in some patients, their ANCS can even improve spontaneously.8–10
When the pressure in the carpal tunnel reaches 20–30mmHg, we can have a mild reduction in epineurial blood flow, this being the first manifestation of the median nerve compression; clinically we will have very mild paresthesia.11 If the pressure in the carpal tunnel reaches 30mmHg the axonal transport becomes impaired. This happens mostly at night, when it is easier that the wrist can be in hyperflexion or hyperextension for longer periods; this is the reason why splints can improve the symptoms, at least partially and/or temporally. If the pressure in the carpal tunnel increases beyond 30mmHg, up to 40mmHg, it is very likely that neurophysiological changes will start developing. At higher pressures, at 60mmHg, we will have epineurial edema and axonal transport block; also intraneural ischemia and motor and sensory block.11 It looks plausible that most patients with intracanal pressures between 20 and 40mmHg, will have symptoms, but likely no abnormal neurophysiological findings, at least during the first periods. The amount of time needed to develop neurophysiological changes in NCS is not established.
A topic that has been discussed widely in the literature is that in the term “NCS”, there are too many procedures included under this umbrella.12 Some groups perform classic electromyogram with needles,13 while others prefer electroneurogram with surface electrodes. The “best-practice” is followed in the choice of test and how to perform them; we kindly refer our readers to the American Association of Neuromuscular & Electrodiagnostic Medicine Guidelines (AANEM).13–15 These guidelines were published in an attempt to unify NCS diagnostic criteria, and even new methods to define new cut-off values have been proposed.15
There is great discrepancy about the usefulness of NCS in the diagnosis of CTS.11,16 Some authors (including many surgeons) believe that NCS are the most objective confirmation of the median nerve entrapment in the carpal tunnel, so they consider them mandatory for accurate diagnosis of the syndrome. In other words, it should be the gold standard in CTS diagnosis.17,18
On the contrary, other authors consider that CTS is a clinical diagnosis because the features of other pathologies are different enough from classical CTS to avoid a false diagnosis. In other words, they consider NCS only as an accessory diagnostic tool, which does not contribute significantly to the diagnosis.19–21
Another argument in favor of avoiding NCS is that the best evidence of effective treatment is actually relief of the symptomatology and not the normalization of NCS. Actually, post-treatment NCS is not performed routinely, especially if the patient has a clinical improvement.
NCS could be helpful in cases when the diagnosis is not clear enough, when symptoms are not typical, in the presence of comorbidities such as peripheral or diabetic neuropathy, cervical radiculopathy or other systemic conditions, when more than one site of compression is suspected, or in failures and complications of surgery. Paradoxically, very little is known about CTS in these comorbidities, as these patients are specifically withdrawn from the great majority of CTS studies.
Atroshi et al.1 published that 23 (18%) of 125 patients with no clinical signs and symptoms of CTS were found to have ANCS of the median nerve at the wrist (systemic disease was excluded). Therefore, we could suppose that patients with systemic disease would have at least 18% (maybe an even higher proportion) of abnormal results. On the other hand, Witt et al.22 reported that 25% of their patients with typical CTS signs and symptoms had NNCS, i.e. about one quarter of these patients had a false negative NCS result. Assuming that a proportion of those patients could have had a very mild CTS (not detected with NCS), another proportion of the patients could have an incorrect clinical diagnosis. We see that there can be false positives and false negatives NCS in CTS diagnoses. The real problem is that we don’t know among this 25% of false negatives, how many of them are “authentic” mild CTS (but not detectable with NCS yet), and how many are “other non-CTS” diagnoses.
In practical terms, we acknowledge that NCS is a time consuming procedure, inconvenient for the patient and relatively expensive.
The classical question that had been raised a long time ago is: should we wait for NCS to show abnormalities before we can treat the patient, and especially before we consider for surgery? Alternatively, could we just send the patient for a LCI (ex juvantibus), as this procedure is much less aggressive, “almost innocuous” and quite affordable, in comparison to surgery?
We agree with most authors, that NCS are essential in cases where there are signs of hypotrophy of thenar eminence, polyneuropathy or multiple nerve compression levels, for cases with unclear clinical features, and whenever medico-legal issues are involved.
Sun et al. illustrated that the longer the duration of the symptoms before surgery, the worse the results.23 In contrast, other authors did not find this correlation between the duration of symptoms and the outcome of surgical treatment.24,25
ObjectivesThe purpose of our study was to compare the clinical response to LCI of two groups of patients with clinically typical CTS from the same population area: one group with NNCS and the other group with ANCS.
Patients and methodsStudy designThis is a prospective, open clinical assay. The study was conducted in accordance with the principles of the Declaration of Helsinki. The Ethics Committee of the University Hospital “Ramón y Cajal” reviewed and approved the study. Written informed consent was obtained from each patient before study enrolment. This manuscript adheres to CONSORT guidelines. The trial registration number of the study is ISRCTN26264638.
Study populationThis study was conducted at the 4th Health Area in Madrid, which comprises about 510,000 people. Inclusion criteria were patients older than 18 years, with suggestive symptoms of CTS (daily nocturnal pain/paresthesias in the median nerve territory) of at least three-month duration, from a primary care setting.
Their general practitioners referred these patients to a special CTS unit (specifically created for this study), at a primary care health center. These patients had not responded to a course of at least two weeks of nonsteroidal anti-inflammatory drugs and splinting.
Exclusion criteria were previous LCI or surgery in the involved wrist, pregnancy, diabetes mellitus, hypothyroidism, inflammatory arthropathy, polyneuropathy, or clinical signs of severe motor impairment (atrophy of thenar eminence and/or muscle weakness).26
All patients were evaluated by the same investigator (DL-P). After a complete clinical history and physical examination, those patients with a clinical suspicion of CTS (pain, tingling, burning or numbness, in the distribution of the median nerve) were invited to participate in the study. An informed consent was obtained, and patients were referred for NCS of both median and ulnar nerves of the affected side, always performed by the same investigator (GdB).26 Using NCS for CTS diagnosis, we followed the method and diagnosis criteria described by Kimura.26 that were either: (1) a motor latency in the median nerve from the wrist to the abductor pollicis brevis muscle that was >2SD above the normal mean (4.2m/s), with a difference with respect to the distal latency of the ulnar nerve that was >2SD above the mean (1.4m/s); or (2) a decrease in the sensory conduction velocity, from the wrist to the third finger, that was >2 SD below the normal mean (44m/s), with a latency difference with respect to the sensory potential of the ulnar nerve, that was >2SD above the mean (0.7m/s). The lower normal value for the amplitude of evoked potentials of the median nerve, considered to be the mean minus 2SD, was 3.5mV for the motor potential and 19μV for the sensory potential.
EndpointsWe used the Visual Analog Scale (VAS) of pain (p-VAS) both before and after the LCI, to ascertain the effect of LCI. No criteria are universally accepted as a proper response to treatment in CTS. In spite of this, as for other rheumatic conditions, we assumed that a 20% improvement over baseline values could be admitted as a clinically significant response to the treatment.26
We were unable to use the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire because it had not been validated in the Spanish population when this study was started.27
TreatmentWhen ANCS was confirmed, the involved wrist entered in another study, and was randomly assigned to surgical decompression or to local corticosteroid injection.26 When NNCS was confirmed, the wrist was included in this current study and was treated with LCI. All the LCI (both from patients with NNCS and ANCS) were performed by the same investigator (DLP) using a standard technique.26
A subcutaneous gauge needle was positioned approximately 1cm proximal to the distal wrist-flexion crease and medial to the palmaris longus tendon. The needle was inserted at a 45-degree angle distally and advanced approximately 1–2cm in depth. We instilled 20mg in 1ml of paramethasone acetonide, beneath the transverse carpal ligament from the ulnar side of the wrist.29 No local anesthetic was added to the corticosteroid. As already these had failed before the local injection was given, no other oral painkillers, anti-inflammatories, or splint were added at this stage. Further details in our previous paper.26 If symptoms did not disappear completely (p-VAS=0), a second infiltration (same as the first one) was administered in a fortnight.
Patients were followed at 3, 6 and 12 months after treatment. Primary outcome was the proportion of patients reaching at least a 20% improvement over baseline values in p-VAS at 12-month follow-up. We compared the NNCS wrists with ANCS treated with LCI (from the wrists randomized to the LCI group of our previous randomized clinical trial), with the NNCS wrists, that were all treated with LCI.
Statistical analysisStatistical signification for continuous variables were established with the Student's “t” test or Mann–Whitney's “U” test in case of normal or non-normal distribution, respectively.
For qualitative variables, we used the normal χ2 test and Yates’ correction when needed. A p<0.05 was considered significant.
ResultsWe included 44 wrists in the NNCS group and 83 in the ANCS (i.e., the wrists that were randomized to the LCI group from our previous trial).26
The mean age was 49 years in the NNCS group and 54 years in the ANCS group (p=0.063). The mean time since onset of symptoms was 26 weeks in the NNCS group and 38 weeks in the ANCS group (p=0.33). No differences between sexes was found. The mean baseline p-VAS was 59mm in the NNCS group and 42mm in the ANCS group (p=0.001) (Table 1). The body mass index was not recorded in our patients.
As explained before, the protocol allowed a second (and last) infiltration (same as the first one), in 2-week time, if symptoms did not disappear completely (p-VAS=0). In the ANCS, 69 of the 82 (one patient rejected the treatment) wrists (84%) needed a second local injection, as p-VAS was not zero. In the NNCS, 36 of the 44 wrists (81.8%) needed a second local injection. As explained above, we excluded patient with previous LCI or surgery in the involved wrist, pregnancy, diabetes mellitus, hypothyroidism, inflammatory arthropathy, polyneuropathy, or clinical signs of severe motor impairment (atrophy of thenar eminence and/or muscle weakness).
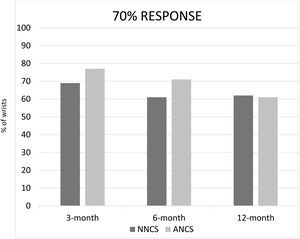
The percentage of patients that reached a response of 20%, 50% and 70% in both groups, NNCS and ANCS, at 3, 6 and 12-month follow-up were recorded26 (Figs. 1–3). There was no statistical significance among all data in both groups, except in the 12-month follow-up, where the NNCS group achieved better results than the ANCS group in the 20% response (p=0.006). There was a trend toward a better 50% response in the 12-month follow-up (Fig. 2).
In terms of quantitative analysis, Table 2 shows the mean and standard deviation of visual analog scale of pain in both groups. Besides a significant reduction in pain from baseline pain in both groups, there was not any statistical significant difference among groups during the follow-up (Table 2).
DiscussionThere was no statistical significance between age or time evolution in both groups (NNCS and ANCS). Nonetheless, both groups had different baseline p-VAS (mean baseline p-VAS was 59mm in the NNCS group and 42mm in the ANCS group, p=0.001). See Table 1.
Our results show a similar clinical response to LCI in CTS patients regardless of NNCS or ANCS. Nonetheless, at 12-month follow-up, the NNCS CTS group did better at 20% pain improvement in the p-VAS, but not at more stringent responses, as 50% or 70% response. There was a trend toward a better 50% response in the 12-month follow-up (Fig. 2). It is probable that with a larger sample, this 50% response in the 12-month follow-up could also reach significant values.
There are very few papers about the clinical response to LCI in clinically typical CTS but with normal NCS (NNCS).11 To our knowledge, studies comparing NNCS CTS patients vs abnormal NCS (ANCS) CTS patients in the same population, all of them being treated with LCI, have not been conducted before.
In most of the medical congresses we attended, one could hear that many clinicians (regardless if general practitioner, rheumatologist, orthopedic, or other specialty), agreed that CTS is mainly diagnosed on clinical grounds (i.e.: nocturnal paresthesias and pain in median nerve distribution), and most of them will perform a LCI, even with NNCS. Despite this, paradoxically, many journals are unlikely to publish any paper regarding CTS treatment without objective features, mainly ANCS. We believe this is the main reason why there are almost no papers with large series of injected NNCS CTS wrists, where we can ascertain the impact of injections in the treatment of this “clinical diagnosed CTS”, but with NNCS.11
Witt et al. studied patients with clinical criteria for CTS and compared NNCS patients with ANCS patients.22 About one quarter of the CTS without confounding neurologic disorders had NNCS with median palmar nerve stimulation. Patients with ANCS were older, heavier and had more clinical features of CTS. NCS could not predict the outcome of conservative management and NCS could not be predicted accurately from clinical features by use of logistic regression models.25 This was especially true in clinically borderline cases. The authors concluded that NCS provide independent information in the evaluation of suspected CTS, especially when fewer clinical criteria are present, but that NCS are not helpful in predicting the outcome of nonsurgical management.22
In a recent study,28 similar results were published; patients with NNCS had similar results than patients with ANCS. Nonetheless, patients with NNCS had a lower satisfaction score than ANCS ones.
Furthermore, another recent study29 reported that high-index suspicion cases of CTS have good surgery results despite NNCS, if they have had a positive response to previous local corticosteroid injection (LCI). The LCI can provide reassurance before proceeding to surgery. Although patients with NNCS and no response to LCI can still respond well to surgery, the surgeon should study thoroughly the clinical history and physical examination, because surgical success decreases with NNCS and failed LCI. These authors suggest that previous response to LCI is an additional diagnostic tool in the management of CTS patients and NNCS.29
We believe that the practically similar results obtained in NNCS and ANCS clinically CTS wrists, support the theory that the clinical symptoms are paramount, and it is likely that ANCS only represent a more severe stage in CTS evolution. At least, at this stage we have some evidence that nerve conduction is impaired enough “that can be documented” in NCS.
It is also well known among clinicians who treat CTS, regardless their specialty, that the degree of severity of NCS do not correlate well with the severity of symptoms. Actually, the most severe CTS, with atrophy of thenar eminence can be also asymptomatic because of denervation.
Our study has several weak points. Despite the fact that the samples are not too small, we cannot consider them as big samples. Likely, with bigger samples, our results could point to more precise conclusions. The longest follow-up was 12 months; it is likely that if longer controls could be done, and NCS could be repeated, this would have given us a more precise idea of how time could affect these wrists.
We consider as strong points that all patients had the NCS of both the median and ulnar nerves of the affected side performed by the same neurophysiologist (GdB) using the same device. All patients were evaluated and injected by the same investigator (DL-P). Our patients were referred from their general practitioners in an outpatient setting, directly to the CTS unit. All our patients were from the same urban area of Madrid. None of them had any work-related or medico-legal issue, as these patients are attended in Madrid Mutual Benefit Society for Work-related Accidents and Occupational Illnesses, not by general practitioners.
After all these considerations, the only statement we can make without any fear of being wrong regarding CTS diagnosis, is that currently there is no written consensus about the real necessity of performing NCS, nor in what kind of patients. All the authors are able to give reasonable arguments for their choice. As is usual in the art of Medicine, the patients’ signs and symptoms combined with the clinical experience of the treating physician, using available evidence will be necessary for approaching each CTS patient. In CTS diagnosis, it is more certain than ever the famous aphorism from Hippocrates: “there are not diseases but rather patients”.
Ultrasonography has been advocated as a very good alternative tool in CTS diagnosis; nonetheless, a review of ultrasonography as a diagnostic tool for CTS is beyond the objectives of our discussion and of our study. Furthermore, it may be that a combination of clinical signs and symptoms, NCS and ultrasonography could give us a much more precise diagnosis of CTS with fewer false positives and negatives,30 but this hypothesis will need further studies.
In summary, our data suggest that LCI are similarly effective in both CTS with NNCS and ANCS. Nonetheless, there is a mild better effect in NNCS than in ANCS at 12-month follow-up.
Data availability statementThe data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ contributionsDLP attended and reviewed the patients, injected all patients, collected data, wrote the paper and is the corresponding author. JLAS conceived, designed, coordinated the study, helped in data analysis and writing the paper. GdBB did the neurophysiological studies of all patients and assisted in the analysis of data. ASO helped in study design and assisted in data collection. IM provided statistical consulting, helped in study design, and did data analysis. All authors discussed the results and commented on the manuscript.
FundingNo funding available.
Conflicts of interestNone declared.
The authors are indebted to (1) Dr. Marie Scully, partner at Abbey House Medical Centre, for her critical review, helpful comments and careful English revision. (2) Dr. Ana Royuela, Unidad de Bioestadística. Instituto de Investigación Sanitaria Puerta de Hierro-Segovia de Arana, for her contribution in statistical analysis of data.