Myasthenia gravis (MG) is an autoimmune disease that affects the neuromuscular junction of the striated muscles. It provokes a fluctuating weakness of the voluntary muscles, mainly due to the direct attack of autoantibodies against the acetylcholine receptor (AChR).1
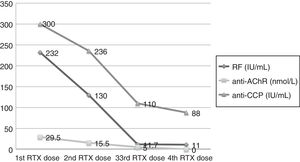
Our patient was a 66-year-old woman, diagnosed with rheumatoid arthritis (RA) in 2012. She was being treated with 20mg/week of methotrexate. After 23 months of good clinical control, her disease became active, and treatment was begun with etanercept at 50mg/week. After 6 doses of etanercept, she came to the emergency department with complete ptosis of right eye with a fluctuating course, that had developed 3 days earlier. She had a limitation of upgaze and, thus, was admitted by the neurology department with suspected ocular MG. During her hospital stay, she underwent brain magnetic resonance imaging and chest computed tomography, both of which were normal. Laboratory tests showed normal erythrocyte sedimentation rate and C-reactive protein, antinuclear antibodies and extractable nuclear antigens were negative, rheumatoid factor was 232IU/mL (normal level 0–14), anti-cyclic citrullinated peptide antibodies >300IU/mL (0–20) and anti-AChR antibodies 29.52nmol/L (0–0.25). Electromyogram revealed an increase in mean jitter, presence of right frontalis muscle blocks and a decrease in the amplitude at rest of facial nerve signal to nasalis muscle, compatible with a postsynaptic neuromuscular transmission defect. With the confirmation of the diagnosis of ocular MG, it was decided to discontinue etanercept. Three weeks after the interruption of etanercept therapy, which was replaced by 30mg/day of prednisone, the patient's neurological status improved. However, over the course of 12 weeks, during which the prednisone dose was being tapered, she developed a polyarticular flare and the ocular symptoms reappeared. It was decided to administer a course of rituximab (2 doses of 500mg separated by 15 days), followed by retreatment (500mg every 6 months), with which control of the joint and neurological symptoms was achieved (Fig. 1).
Monitoring of the levels of rheumatoid factor (RF), anti-acetylcholine receptor (anti-AChR) and anti-cyclic citrullinated peptide (anti-CCP) antibodies in terms of the clinical response to rituximab (RTX). There was a progressive decrease in anti-AChR which was not detectable following the fourth dose.
Approximately 5% of the population has one or more autoimmune diseases, and the prevalence is highest in middle-aged women.2 Patients who have an autoimmune disease are more susceptible to developing a second one. Myasthenia gravis is associated with RA in up to 4% of the patients.3
This association may be due to immunological factors that favor the activation of autoreactive B and T cells, epigenetic factors and genetic susceptibility to certain groups of genes, particularly those belonging to the major histocompatibility complex.3 Thus, if we consider that the overlap of autoimmune disorders is a reflection of the existence of common pathogenic mechanisms, the therapeutic approach should be the same.
Anti-tumor necrosis factor alpha (TNFα) agents have favored the treatment and prognosis of rheumatic diseases like RA, in addition to being employed in other autoimmune diseases. In fact, etanercept has produced satisfactory results in the treatment of refractory MG,4 with no effect on serum anti-AChR levels or changes in circulating TNFα. Paradoxically, the literature includes the report of 1 case of MG in a patient with RA being treated with etanercept,5 in whom the discontinuation of the drug resulted in the improvement of the myasthenic signs.
Although there is no clear causal association, the development of neurological disorders, especially demyelinating diseases (Guillain-Barré syndrome, multiple sclerosis, mononeuritis or chronic demyelinating polyneuropathy6,7), has occasionally been related to anti-TNFα agents. Should they occur, the main recommendation is to discontinue the treatment.
In the present case, there existed the possibility of there being an association between 2 autoimmune diseases or, on the other hand, a probable adverse effect of etanercept. The decision was made to interrupt the drug and begin treatment with rituximab, the utility of which has been reported in the treatment of refractory MG.8 Its mechanism of action provokes a depletion of B cells and the resulting reduction in antibodies, enabling the clinical control of both diseases. The doses administered are generally 375mg/m2, with retreatment every 6 months given the half-life of this agent,9 although there is still no consensus concerning the guidelines for its administration in MG.
Please cite this article as: Novella-Navarro M, Salvatierra-Ossorio J, Muñoz-Gómez MM, Pavo-Blanco M. Artritis reumatoide y miastenia gravis ocular: efectividad del rituximab en el manejo de ambas enfermedades. Reumatol Clin. 2018;14:179–180.