ANCA-associated vasculitides (AAV) are chronic autoimmune diseases characterized by inflammation and destruction of small vessels. Rituximab is now licensed for use as a remission-induction agent in the treatment of these disorders. During recent years, several non-controlled studies have suggested that rituximab may be of value in maintaining disease remission in AAV. In these series, 3 techniques have been tried: “watch-and-wait”, repeated cycles in fixed intervals, or administration based on proposed biomarkers. More importantly, the results of the MAINRITSAN trial showed that this anti-CD20 agent is superior to azathioprine for preventing major relapses in AAV. This review summarizes current information regarding the effectiveness, timing, dosing, duration and safety of rituximab as a valid option for remission maintenance.
Las vasculitis asociadas a ANCA son enfermedades autoinmunes crónicas que se caracterizan por inflamación y destrucción de vasos de pequeño tamaño. El rituximab es un tratamiento efectivo para la fase de inducción de estas patologías. Durante los últimos años, varios estudios no controlados han reportado que también es eficaz durante la fase de mantenimiento terapéutico. En estas series, el fármaco se administró solo durante las recaídas, a intervalos fijos o sobre la base en cambios en algunos biomarcadores. Los resultados del estudio MAINRITSAN mostraron que el rituximab es superior a la azatioprina como terapia de mantenimiento en estas enfermedades. Este trabajo de revisión resume la información más reciente sobre el uso de rituximab como opción para la fase de mantenimiento de las vasculitis asociadas a ANCA, detallando su efectividad, los diversos protocolos de administración, el perfil de seguridad y el uso potencial de biomarcadores para guiar el tratamiento.
Vasculitides associated with antineutrophil cytoplasmic antibodies (ANCA) are a group of multisystemic diseases characterized by necrotizing inflammation of small vessels.1 The term ANCA-associated vasculitides (AAV) includes granulomatosis with polyangiitis (GPA, Wegener's granulomatosis), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA, Churg-Strauss syndrome).1
Rituximab (RTX), a chimeric monoclonal antibody that integrates the anti-CD20 variable region of a murine immunoglobulin (Ig) with the constant region of a human IgG1, is one of the therapeutic alternatives approved for the induction phase in both GPA and MPA.2 In recent years, a number of studies have evaluated the use of RTX as maintenance therapy in these vasculitides.
In this review, we examine in detail the available information on the efficacy of RTX in maintaining remission in AAV. For this purpose, we conducted a search in the database of the United States National Library of Medicine (PubMed) for articles in English or Spanish published between January 2001 and January 2015. The terms, included in both languages, were: ANCA, vasculitis, granulomatosis with polyangiitis, microscopic polyangiitis and Wegener's granulomatosis in combination with rituximab, anti-CD, maintenance therapy and biological therapy. The relevant articles were obtained in their complete version and were reviewed and analyzed by both authors. We also identified noteworthy information in the abstracts of international rheumatology meetings held over the last 5 years. This report does not include the use of RTX in EGPA, which was recently reviewed in this journal.3
Maintenance Therapy for Vasculitides Associated With Antibodies Against Neutrophil Cytoplasmic AntigensThe treatment of GPA and MPA consists of two different stages, an induction phase (with intensive immunosuppression) and a maintenance phase. The purpose of the latter is to prevent relapses and limit drug toxicity.
Despite the fact that initial treatment with cyclophosphamide (CPM), RTX or methotrexate (MTX) in combination with high-dose glucocorticoids (GC) achieves partial or complete remission in 75%–90% of the patients with AAV,4,5 11%–57% of them have relapses while they are being treated with azathioprine (AZA) or MTX alone.6–8 Although the majority are mild, some can be severe, and may provoke additional organ damage, permanent functional sequelae or even death. In addition to the complications inherent to the disease, 40% of the patients develop adverse effects related directly to the therapy employed, for example, osteoporosis or cardiovascular complications.9,10
With this scenario, the need to improve certain aspects of maintenance therapy is evident. Especially important among the unresolved matters are: (1) optimization of dose reduction and duration of therapy with the drugs employed, as most of the relapses occur during the tapering of GC and/or immunosuppressive agents (IS) or once they have been discontinued11; (2) early identification of patients at high risk for relapse, for example, those with GPA, upper respiratory tract disease, PR3-ANCA positivity, nasal colonization by Staphylococcus aureus or persistence of ANCA when AZA is initiated12–16; (3) development of individualized treatments in which not only the clinical and ethnical differences are taken into account, but the histological, serological and genetic associations as well11,17; and (4) evaluation of new drugs, such as abatacept (clinicaltrials.gov, NCT02108860), belimumab (BREVAS study, NCT01663623) or RTX, the drug being evaluated here.
The Basis for the Use of Rituximab in Vasculitides Associated With Antibodies Directed Against Neutrophil Cytoplasmic AntigenThe evidence at different levels indicates that the B lymphocytes play a very important role in AAV: (1) the number of activated B cells is associated with the disease activity and severity18; (2) ANCA, antibodies possibly involved in the pathogenesis of these vasculitides, are produced by B lymphocytes19,20; (3) in nasal, lung and orbital biopsies from patients with GPA, self-reactive B cells have been identified, adjacent to PR3-ANCA-positive cells, which form structures similar to lymphoid follicles that disappear after treatment with RTX21–24; and (4) B lymphocytes are the main target of CPM, a drug that is essential in the treatment of these vasculitides.25
The binding of RTX to CD20 provokes the rapid and sustained depletion of premature and mature B lymphocytes (reducing the plasma cell precursors) through diverse mechanisms, which include antibody-mediated cellular cytotoxicity (mediated by macrophages and natural killer cells), complement-mediated lysis, induction of apoptosis and sensitization to GC and cytotoxic agents.26 Moreover, the drug is capable of inhibiting the interaction between self-reactive B and T cells and increasing the regulatory T lymphocyte population.27–30
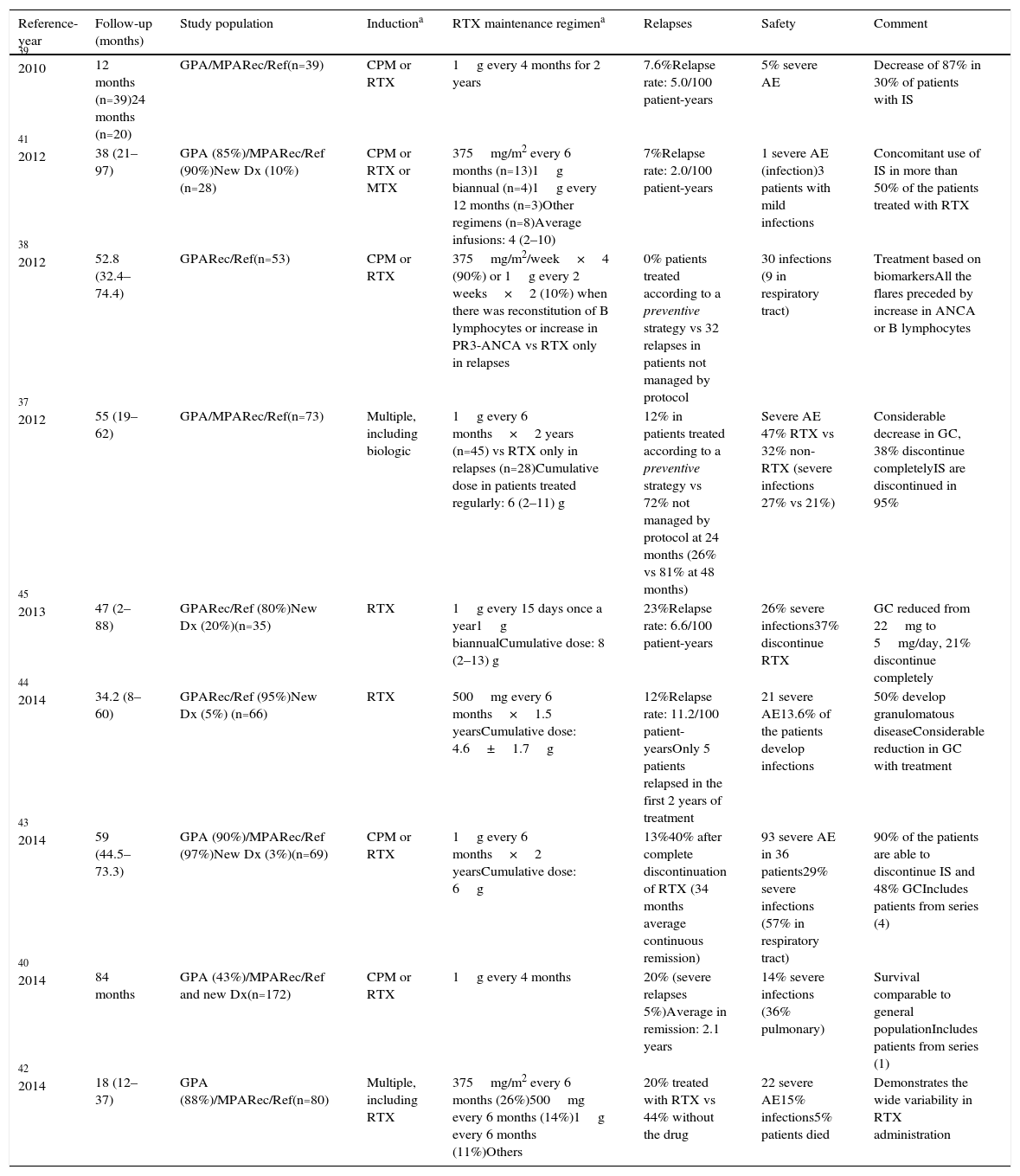
Rituximab as Maintenance TherapyInformation on the use of RTX as maintenance therapy derives from the MAINRITSAN (Maintenance of Remission using Rituximab in Systemic ANCA-associated vasculitis) trial, a recent randomized clinical trial, as well as from certain observational studies (Table 1).
Studies Related to Maintenance Therapy With Rituximab (Fixed Intervals).
| Reference-year | Follow-up (months) | Study population | Inductiona | RTX maintenance regimena | Relapses | Safety | Comment |
|---|---|---|---|---|---|---|---|
| 39 2010 | 12 months (n=39)24 months (n=20) | GPA/MPARec/Ref(n=39) | CPM or RTX | 1g every 4 months for 2 years | 7.6%Relapse rate: 5.0/100 patient-years | 5% severe AE | Decrease of 87% in 30% of patients with IS |
| 41 2012 | 38 (21–97) | GPA (85%)/MPARec/Ref (90%)New Dx (10%)(n=28) | CPM or RTX or MTX | 375mg/m2 every 6 months (n=13)1g biannual (n=4)1g every 12 months (n=3)Other regimens (n=8)Average infusions: 4 (2–10) | 7%Relapse rate: 2.0/100 patient-years | 1 severe AE (infection)3 patients with mild infections | Concomitant use of IS in more than 50% of the patients treated with RTX |
| 38 2012 | 52.8 (32.4–74.4) | GPARec/Ref(n=53) | CPM or RTX | 375mg/m2/week×4 (90%) or 1g every 2 weeks×2 (10%) when there was reconstitution of B lymphocytes or increase in PR3-ANCA vs RTX only in relapses | 0% patients treated according to a preventive strategy vs 32 relapses in patients not managed by protocol | 30 infections (9 in respiratory tract) | Treatment based on biomarkersAll the flares preceded by increase in ANCA or B lymphocytes |
| 37 2012 | 55 (19–62) | GPA/MPARec/Ref(n=73) | Multiple, including biologic | 1g every 6 months×2 years (n=45) vs RTX only in relapses (n=28)Cumulative dose in patients treated regularly: 6 (2–11) g | 12% in patients treated according to a preventive strategy vs 72% not managed by protocol at 24 months (26% vs 81% at 48 months) | Severe AE 47% RTX vs 32% non-RTX (severe infections 27% vs 21%) | Considerable decrease in GC, 38% discontinue completelyIS are discontinued in 95% |
| 45 2013 | 47 (2–88) | GPARec/Ref (80%)New Dx (20%)(n=35) | RTX | 1g every 15 days once a year1g biannualCumulative dose: 8 (2–13) g | 23%Relapse rate: 6.6/100 patient-years | 26% severe infections37% discontinue RTX | GC reduced from 22mg to 5mg/day, 21% discontinue completely |
| 44 2014 | 34.2 (8–60) | GPARec/Ref (95%)New Dx (5%) (n=66) | RTX | 500mg every 6 months×1.5 yearsCumulative dose: 4.6±1.7g | 12%Relapse rate: 11.2/100 patient-yearsOnly 5 patients relapsed in the first 2 years of treatment | 21 severe AE13.6% of the patients develop infections | 50% develop granulomatous diseaseConsiderable reduction in GC with treatment |
| 43 2014 | 59 (44.5–73.3) | GPA (90%)/MPARec/Ref (97%)New Dx (3%)(n=69) | CPM or RTX | 1g every 6 months×2 yearsCumulative dose: 6g | 13%40% after complete discontinuation of RTX (34 months average continuous remission) | 93 severe AE in 36 patients29% severe infections (57% in respiratory tract) | 90% of the patients are able to discontinue IS and 48% GCIncludes patients from series (4) |
| 40 2014 | 84 months | GPA (43%)/MPARec/Ref and new Dx(n=172) | CPM or RTX | 1g every 4 months | 20% (severe relapses 5%)Average in remission: 2.1 years | 14% severe infections (36% pulmonary) | Survival comparable to general populationIncludes patients from series (1) |
| 42 2014 | 18 (12–37) | GPA (88%)/MPARec/Ref(n=80) | Multiple, including RTX | 375mg/m2 every 6 months (26%)500mg every 6 months (14%)1g every 6 months (11%)Others | 20% treated with RTX vs 44% without the drug | 22 severe AE15% infections5% patients died | Demonstrates the wide variability in RTX administration |
All the reported series are observational cohorts from a single center, except (9) (multicenter retrospective).
AE: adverse events; CPM: cyclophosphamide; Dx: diagnosis; GC: glucocorticoids; GPA: granulomatosis with polyangiitis; IS: immunosuppressive agents; MPA: microscopic polyangiitis; MTX: methotrexate; Rec/Ref: recurrent/refractory; Ref: reference; RTX: rituximab.
MAINRITSAN is the first and only randomized study to compare RTX and AZA as maintenance therapy in patients with GPA (n=87), MPA (n=23) or renal-limited vasculitis (n=5).31 The study (controlled, open and multicenter) included 115 patients (80% with new diagnosis, 20% with recurrent disease) in complete remission after induction therapy with GC and CPM. The maintenance protocol was based on the administration of prednisone (approximately 5mg/day for at least 18 months) in combination with AZA (n=58, 2mg/kg/day for 1 year, 1.5mg/kg/day for 6 months and 1.0mg/kg/day for the last 4 months) or RTX (n=57, 500mg initially on days 0 and 14 and, thereafter, every 6 months, for a total of 5 applications). The percentage of patients with severe relapses (recurrence or worsening of the vasculitis with manifestations that were life-threatening or affected an essential organ, like the brain or heart) after 28 months of follow-up (primary endpoint) was 5% (n=3) among patients treated with RTX and 29% (n=17) among those treated with AZA, demonstrating a clear superiority of the monoclonal antibody (P=.002). Among the patients who relapsed with AZA, in 8 it occurred during the first year; in 2, between months 12 and 22; and in 7, after the complete discontinuation of the medication (months 24–28). In the case of RTX, there was 1 relapse per month in months 8, 22 and 24. With respect to minor relapses, there were no significant differences between the two groups in terms of the rate of adverse events or the incidence of infections.
Although the results of the study with RTX are very promising, with one of the lowest rates of relapse reported in these vasculitides, it strikes us as interesting that 41% of the flares recorded in the AZA group occurred when the patients were no longer receiving the medication, possibly causing the therapeutic effect of the drug to be underestimated. However, the protocol for maintenance with AZA is similar to that utilized previously in two large-scale clinical trials involving the same type of patients, and the results of which are similar to those observed in the MAINRITSAN trial.5,8 As occurs with other IS,32 treatment with AZA or RTX for a longer period of time than that evaluated in the MAINRITSAN trial could be related to a higher rate of sustained remissions, a possibility that should be examined in clinical trials with longer follow-up times.
In short, the MAINRITSAN trial showed that the combination of low doses of GC and RTX at fixed intervals (every 6 months, which is the average duration of the therapeutic effect33) can be considered a valid, effective and safe alternative for maintenance of remission in patients with AAV, mainly those with newly diagnosed GPA. At the present time, two clinical trials to evaluate RTX as maintenance therapy are being developed. The first, MAINRITSAN 2 (clinicaltrials.gov NCT01731561), will compare two strategies for RTX administration (500mg at fixed intervals of 6 months vs 500mg whenever increases in CD19+ lymphocytes or ANCA are detected). The other study, RITAZAREM (Rituximab Vasculitis Maintenance Study) (NCT01697267), will evaluate patients with refractory AAV receiving RTX (1g every 4 months) or AZA for 2 years.
Observational StudiesThe studies that are to be reviewed below disclose certain differences and limitations that should be taken into account for a correct interpretation of the conclusions derived from them. These series evaluated heterogeneous populations, the dose and regimen of RTX administration varied, and there are differences in the reported maintenance protocols, as the drug was utilized: (1) at fixed intervals in a so-called prevention strategy; (2) depending on serological markers, such as an increase in the ANCA level or B lymphocyte reconstitution; or (3) only at the time of relapses.
Rituximab at Fixed Intervals (Preventive)This strategy has become that most widely used in recent years, and rates of sustained remission (follow-up of 36–48 months) of 74%–100% have been reported (in comparison with the 19%–56% in patients not treated regularly with RTX).34–45Table 1 summarizes the features of the major studies.
Although the doses and intervals vary, the administration of 1g every 4–6 months for 2 years is the regimen that is most frequently used.37,39–42 In some protocols, RTX was continued for more than 24 months, meaning that these patients may have received ≥10 cycles of the drug.38,40,43 In contrast to the MAINRITSAN randomized clinical trial, in the great majority of the observational studies, the predominant study population consisted of patients with recurrent or refractory GPA (mean age of 40.5–52 years), and the number of newly diagnosed patients was lower.37,41,44
With this form of administration, the average time between relapses and the first and last doses of the monoclonal antibody was 11–29 months and 9–15 months, respectively.34,37,42–45 Interestingly, a study with an extensive follow-up period (5 years) demonstrated that, after discontinuation of the medication (24 months), 50% of the patients remained in sustained remission, whereas the remainder relapsed 3 years after the last dose of RTX.43
With regard to drug toxicity, although the cumulative dose of the drug is greater in these patients, the rate of adverse effects (including infections) is similar to that reported in studies in which RTX was administered only during relapses.34–45 However, it should be stressed that one limitation of preventive maintenance with RTX is the risk of overtreatment of those patients (approximately 40%) who have prolonged remission after a single cycle of induction with RTX.46 Unfortunately, to date there is no way to identify those patients.
Another important datum derived from these studies is that, in the majority of the reported patients, the preventive use of RTX permits the reduction of the prednisone dose (initially 20–30mg/once daily to <5mg/once daily).34,40,41,43,44 Moreover, 70%–90% of the patients treated were able to completely discontinue all other immunosuppressive therapy (mycophenolate mofetil, AZA or MTX) and 21%–48% were able to discontinue treatment with GC.37,39,41,43,45
In this respect, it is important to point out the adjuvant role that the classical IS could play in maintenance therapy with RTX, mainly in patients with recurrent or refractory disease. The administration of AZA, MTX, leflunomide or mycophenolate mofetil in combination with RTX as maintenance therapy has been reported in a number of uncontrolled studies.39,40,44,45,47 A study with a considerable number of GPA patients (n=89) reported that, after 2 years of follow-up, those patients treated with AZA or MTX in combination with RTX had a significantly lower rate of relapses than those treated with RTX alone (55% vs 70%, P=.04).47 These results contrast with those of the subanalysis of 40 patients from the series of Rhee et al.,39 in which the systematic discontinuation of the IS prior to starting treatment with RTX was not associated with an increased frequency of flares. With respect to the therapeutic complications, some studies have reported that the concomitant use of mycophenolate mofetil and, mainly, CPM is related to a higher predisposition toward severe infections,45 whereas, in other series, there was no increase in the number of adverse events.47 At the present time, the available data do not lead to a clear conclusion about the adjuvant use of IS with RTX; however, it is advisable to avoid the combination of the latter with CPM at full doses, due to the increase in the number of severe adverse events.48
Rituximab Based on Biomarker LevelsA retrospective study of GPA patients (n=53) with refractory disease (40% granulomatous activity, 60% vasculitic) compared the administration of RTX only in the case of new relapse (36%) with an individualized protocol (64%) involving infusion of the drug on the basis of the increase in PR3-ANCA or B lymphocytes in serial measurements.38 At the end of the follow-up period (median 4.4 years, interquartile range 2.7–6.2), there were no episodes of reactivation among the patients treated on the basis of the change in ANCA titer or B lymphocyte repopulation (average of 4 cycles of RTX received) in contrast to 32 relapses (relapse rate: 13.8/100 patient years) in the group of patients in which the drug was not administered on a regular basis.
Rituximab in RelapsesThe follow-up of the patients treated with a single RTX cycle in the RAVE (Rituximab in ANCA-associated vasculitis) trial demonstrated that, although the results with this approach are similar to those obtained with the CPM/AZA combination, it is not sufficient to maintain prolonged remission, as approximately 60% of the patients will relapse during follow-up (18 months).13,46
In the light of this evidence, at some centers, retreatment with RTX was adopted as a therapeutic measure only in cases of relapse, and it was observed that the drug (at the dose for lymphoma or rheumatoid arthritis [RA]) is usually equally as effective (and safe) as the initial anti-CD20 therapy.23,36,49 In the open phase of the RAVE trial,5,50 26 patients (92% GPA) received RTX for severe relapses between month 6 and month 18 of the study, and remission was achieved in approximately 90% of the cases (50% were also able to discontinue the concomitant use of GC). These studies have also shown that RTX is more effective than CPM in maintaining remission in patients with previous recurrent disease.46
Although treatment of relapses with RTX appears to be effective, this approach to the administration has the disadvantage that the patients are exposed to the damage derived from the disease, and possibly to higher cumulative doses of prednisone (a drug that is usually increased as an initial measure). Moreover, given that RTX is usually administered full-dose for induction, this could entail an additional economic cost as compared with single, smaller doses administered once or twice a year, as in the prevention protocols.
Predictors of Relapses Following Treatment With RituximabOne persistent question is whether there are biomarkers capable of accurately predicting which patients will relapse during treatment with RTX. Up to now, conclusions based on the evaluation of the two laboratory tests most widely examined (B lymphocyte and ANCA levels) are quite unclear,36,39,45,51 with publications that argue in favor38,49,52,53 and against36,37 their utility.
Determination of Antineutrophil Cytoplasmic AntibodiesIn the vast majority of the reported series, it has not been possible to establish a relationship between the change in ANCA levels and the development of recurrence.5,34,36,37,39,41,44,45,50,54–56 In these studies, the percentages of patients who became ANCA-positive or in whom the levels increased, and who had relapses or remained in remission ranged from 33% to 77% and from 38% to 66%, respectively, which shows that the sensitivity of this variable is too low to serve as the basis for decisions on maintenance therapy. The only study that found an association between ANCA levels and recurrences reported that seroconversion to ANCA positivity during the post-RTX period was associated with a 7-fold higher risk of having severe relapses.43
With respect to the specificity of ANCA, it has recently been reported that the presence of PR3-ANCA, but not a change in the levels of these antibodies, was related to higher long-term risk of relapses.37,43,46,50
One important conclusion derived from the RAVE extension studies is that the possibility of RTX-treated patients having a relapse is extremely low as long as the assays for ANCA are negative and B lymphocytes remain undetectable in peripheral blood.46,50 In fact, only 1 of the 76 patients included in these studies had a flare while these two markers were below the level of detection.
B LymphocytesAs in the case of ANCA, the levels of these cells in peripheral blood have not been shown to be an accurate enough test for the prediction of flares.31,37,39,41,44,56 Depending on the study analyzed, the reported finding is that approximately 50% of the relapses occur prior to B cell repopulation, that 44%–88% of the patients who relapse have detectable B lymphocytes, and that between 25% and 66% of those patients in whom normal levels of B lymphocytes are detected do not have relapses.5,34,36,37,45,46 Moreover, the time between B cell reconstitution and the development of relapses is not constant either; some patients relapse coinciding with lymphocyte repopulation, whereas in others, it may take some time (average 80±85 days).57
One of the few series in which a good correlation was found between AAV reactivation and B lymphocyte repopulation reported that the development of flares (n=32) was always preceded by the detection of these cells in peripheral blood, with a median between the two events of 8.5 months.38 Interestingly, in this report, all the recurrences were preceded by or associated with an increase in PR3-ANCA.
A recent report found that those patients in whom B lymphocytes were detectable within the first year after treatment with RTX had shorter periods of remission than those in whom repopulation took over 12 months.43
The possible hypotheses to explain the poor correlation between the number of B lymphocytes and the AAV activity include: (1) the existence of niches or “sanctuary sites” for self-reactive B cells in tissues or sites of active inflammation (mainly granulomatous)24; these cell groups would be protected from the pharmacological action of RTX, remaining active before an increase in lymphocytes was observed in peripheral blood21,58; (2) changed dynamics in B cell levels due to a synergic effect between RTX and other conventional IS, such as AZA or CPM, that are frequently administered to patients with resistant or refractory disease59; (3) racial differences between the populations studied, as the elimination of the B lymphocytes depends not only on the RTX dose, but on polymorphisms in the cell surface receptors (FcγRIIIa)60; and (4) differing definitions from one study to another, with varied absolute values for peripheral blood B cells and diverse phenotypes evaluated (CD19+ or CD20+), as a consequence of the use of detection techniques (flow cytometry) with greater or lesser sensitivity.36
New BiomarkersA recent publication stated that the detailed phenotypic analysis of subpopulations of B lymphocytes might have the potential to predict relapses. In a study of 54 patients with AAV, it was observed that when <30% of the B lymphocyte repopulation was constituted by CD5+ cells (possibly regulatory B cells), the time to the first recurrence was shorter. This cell subpopulation also correlated negatively with the general disease activity.61 Similarly, it has been reported that, when the B lymphocyte reconstitution is composed mainly of memory cells, there is an increased risk of recurrences.53 This is similar to what occurs in systemic lupus erythematosus, in which repopulation with a higher proportion of CD27+ B lymphocytes (memory cells) has been related to a higher risk of flares.62,63
SafetyThe experience accumulated in the treatment of hematologic neoplasms and RA indicates that RTX has a good margin of safety. The situation in AAV appears to be similar, although the long-term safety of repeated RTX administration has yet to be established, as the majority of the studies have a relatively short follow-up period.
In maintenance studies, anti-CD20+ therapy provoked adverse effects in 13%–60% of the patients,37,42,43 with severe infections, hypogammaglobulinemia, severe neutropenia or a lack of efficacy being the main causes of discontinuation, temporary or definitive, of the drug.44,45
Reactions Associated With the Administration of RituximabMild allergic reactions are the adverse effect most frequently observed (10%–50% of the patients).33,64,51 They usually occur within the first 24h after treatment, and the manifestations are fever, chills, hyper- or hypotension, fatigue, headache, erythema, urticaria, nausea and/or vomiting.33,51
InfectionsThese are the most important complications, and mild or severe infections are reported in 7%–50% of the patients (incidence of 2.6–15.5/100 patient years, similar to that of CPM).5,36,37,42,46,49,50,56,57,65–67 The most common infections are those of the respiratory tract (mainly of viral origin) and the urinary tract, followed by mucocutaneous infections (herpes simplex, herpes zoster and candidiasis).33,36,37,41,46,49,50,65 Although the majority of these episodes are mild, 14%–27% are severe and 3%–29% of the patients develop chronic infections.36,42–45 Infections due to opportunistic microorganisms are rare, and RTX is a safe alternative in patients with a history of tuberculosis.38,39,68
The reported risk factors for the development of severe infections in these patients are high cumulative doses of CPM and the combination of RTX with other cytotoxic drugs.45,69 Although the presence of neutropenia or hypogammaglobulinemia could be associated with increased susceptibility to infectious complications, at present, there is no convincing evidence to prove this.37,38,44
HypogammaglobulinemiaThis adverse effect has been reported in 26%–41% of the patients at some point during maintenance with RTX, but is a transient and self-limiting event in more than 50% of the cases.37,43,70
In the majority of the patients, the most marked decrease in the immunoglobulin level was observed during the initial treatment cycles (60% of all the episodes occurring with total doses ≤2g and 70% of those with doses ≤6g).71
Although hypogammaglobulinemia is usually mild to moderate, approximately 7% of the patients have a severe and/or permanent deficiency, requiring discontinuation of the drug and its substitution by intravenous gammaglobulin.43,44,57,51,72 In this respect, this adverse effect has not been directly related to an increase in the development of severe infections,37,38,44 although it could be a contributing factor in patients with other predisposing conditions.73
Among the risk factors that have been associated with the presence of hypogammaglobulinemia, we point out a high cumulative dose of CPM (prior to the initiation of RTX), low baseline immunoglobulin and CD4+ levels, and the concomitant use of the monoclonal antibody with cytotoxic drugs.57,70 Interestingly, some studies have related hypogammaglobulinemia to the number of RTX cycles.74,75 However, this finding has not been corroborated in series with longer follow-up periods.57,51,70,71
Late-onset NeutropeniaThis develops in 3%–5% of the patients and is generally transient.5 In the majority of the cases, it has no effect on the patient's clinical status, although there may be cases in which the use of a colony-stimulating factor is required.40,44,76,77
Other Adverse EffectsThe published literature includes cases of pancytopenia,33,39 macular edema78 and severe pulmonary complications (interstitial disease) in patients with AAV.48 Moreover, there are reports of progressive multifocal leukoencephalopathy in patients with systemic lupus erythematosus and RA,79,80 and a case (unpublished) of this complication in AAV has been detected.48
ConclusionsRituximab has been shown to be effective and safe for use as maintenance therapy in AAV, both in recently diagnosed disease and in patients with frequent relapses.48 On the basis of the information published to date, the administration of 500mg at fixed intervals (twice a year) could be the recommended mode of administration, because of its marked efficacy and safety. However, lower doses may also prove to be effective in the maintenance of remission, as has been reported in other diseases,81,82 or at wider intervals between applications. This could be guided with the utilization of more reliable biomarkers, as well as the individual response to the treatment.
It still remains to be determined whether the drug is more effective in specific groups, for example, in women of childbearing age or patients susceptible to frequent relapses or with a higher risk of infections. The optimal duration of maintenance with RTX should also be studied. In this respect, and by analogy with conventional treatment, a period of between 18 and 24 months is recommended, although, at the present time, there is nothing conclusive regarding this question.48 Finally, we still have yet to know what effect cumulative doses can have and the economic impact of different administration protocols.83
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Alba MA, Flores-Suárez LF. Rituximab como terapia de mantenimiento en las vasculitis asociadas a ANCA: ¿cómo, cuándo y por qué? Reumatol Clin. 2016;12:39–46.