A case is presented of a protein-losing enteropathy (PLE) as the initial manifestation of systemic lupus erythematosus (SLE) in a 17 year-old female patient, who presented with ascites, edema and hypoalbuminemia. The diagnosis of SLE was based on the presence of: malar rash, oral ulcers, thrombocytopenia, antinuclear antibodies, IgM anticardiolipin antibody, and lupus anticoagulant. Renal and liver diseases were ruled out. The PLE diagnosis was confirmed with fecal alpha 1-antitrypsin clearance. The PLE was refractory to different lines of immunosuppressive agents like glucocorticoids, cyclophosphamide, azathioprine, and cyclosporine, showing a satisfactory and sustained response with rituximab, allowing steroid sparing and long term remission.
Se presenta un caso de enteropatía perdedora de proteínas (EPP)como manifestación inicial del lupus eritematoso sistémico (LES). Se trata de una paciente de sexo femenino, de 17 años de edad, que consulta por síndrome ascítico edematoso e hipoalbuminemia. El diagnóstico de LES se estableció por la presencia de: rash malar, úlceras orales, trombocitopenia, anticuerpos antinucleares, anticardiolipinas IgM y anticoagulante lúpico positivos. Se descartó el compromiso renal y hepático como causa de hipoproteinemia. El diagnóstico de la pérdida enteral de proteínas se realizó con el Clearance de alfa 1 antitripsina. La EPP fue refractaria a distintas líneas de inmunosupresores, como corticoides, ciclofosfamida, azatioprina y ciclosporina, presentando respuesta satisfactoria y sostenida a rituximab, lo que posibilitó la reducción de corticoides y la remisión de la enfermedad por tiempo prolongado.
Gastrointestinal manifestations are common in systemic lupus erythematosus (SLE). Nonspecific symptoms such as anorexia, nausea, vomiting and diarrhea are observed in up to one third of the patients with active disease. Ascites, peptic ulcer disease and dysphagia develop in 8–12%, 6% and 1–7.3% of the cases, respectively. Abdominal pain, present in 8–37% of the patients, may be a sign of a complication, such as mesenteric vasculitis, or may be attributable to causes unrelated to lupus, such as irritable bowel syndrome.1 Protein-losing enteropathy (PLE) is an uncommon clinical manifestation associated with SLE but, in some cases, is the presenting sign.2 The prevalence is around 2–3%.3,4 It is characterized by the presence of hypoalbuminemia secondary to gastrointestinal protein loss. We present the case of a patient with SLE associated with PLE refractory to conventional immunosuppressive therapy with a satisfactory response to rituximab (RTX).
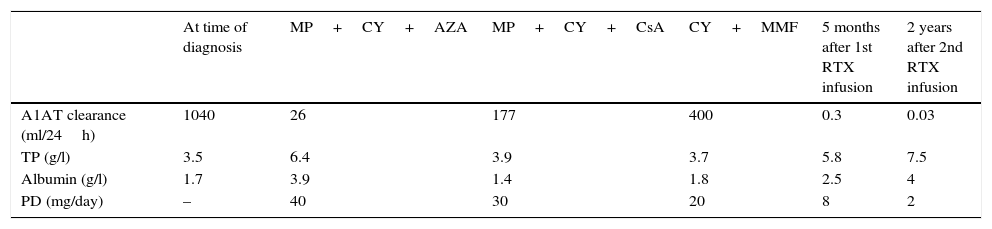
Case ReportThe patient was a 17-year-old woman, who was admitted to the hospital for the study of recent-onset ascites. She had a 3-year history of episodes of chronic abdominal pain and diarrhea. The physical examination revealed malar rash, polyarthritis, alopecia, oral ulcers, anasarca and pleural effusion. The most notable analytical findings were: hemoglobin 11g/dl, platelets 67000/mm3, total protein concentration 35g/l, albumin 17g/l, triglycerides 800mg/dl, erythrocyte sedimentation rate 120mm/1h, C3: 58mg/dl (90–180mg/dl), C4: 13mg/dl (10–40mg/dl), normal urinary sediment and negative test for 24-h urinary protein excretion. Antinuclear antibodies (1/640 dense fine speckled pattern), anti-DNA, anti-Sm, anti-Ro and anti-La were negative; IgM anticardiolipin antibodies, at 36MPL units/ml (reference values [RV] 15–20MPL units/ml), and lupus anticoagulant were positive. Systemic lupus erythematosus was diagnosed on the basis of the 1982 criteria of the American College of Rheumatology. Renal and hepatic compromise were ruled out as the cause of hypoproteinemia. Coproculture, examination of stool for parasites and tests for IgA and IgG antibodies to transglutaminase, IgA and IgG antibodies to deamidated gliadin peptide and IgA antibodies to endomysium were negative. In the study of fecal material, alpha-1 antitrypsin (A1AT) clearance was 1040ml/24h (RV<30ml/24h). Upper gastrointestinal video endoscopy revealed marked edema in the second segment of the duodenum, which resulted in the swelling of the duodenal flexures. Duodenal biopsies disclosed chronic nonspecific duodenitis with a conserved crypt:villus ratio and chorionic edema. Video colonoscopy provided no evidence of lesions and the biopsies showed nonspecific changes. Protein-losing enteropathy associated with SLE was diagnosed, and treatment was begun with methylprednisolone (MP) in intravenous (iv) pulses of 1g/day for 3 days and 6 doses of 500mg of cyclophosphamide (CY) at 2-week intervals, followed by 150mg/day of azathioprine (AZA) and 400mg/day of hydroxychloroquine; the doses of corticosteroids were tapered to 20mg/day. Two months after the start of treatment, remission was achieved and the patient remained asymptomatic for 24 months, during which she continued to take 150mg/day of AZA as maintenance treatment. Table 1 shows the laboratory parameters and response to treatment.
Changes in Laboratory Parameters and Treatment.
| At time of diagnosis | MP+CY+AZA | MP+CY+CsA | CY+MMF | 5 months after 1st RTX infusion | 2 years after 2nd RTX infusion | |
|---|---|---|---|---|---|---|
| A1AT clearance (ml/24h) | 1040 | 26 | 177 | 400 | 0.3 | 0.03 |
| TP (g/l) | 3.5 | 6.4 | 3.9 | 3.7 | 5.8 | 7.5 |
| Albumin (g/l) | 1.7 | 3.9 | 1.4 | 1.8 | 2.5 | 4 |
| PD (mg/day) | – | 40 | 30 | 20 | 8 | 2 |
A1AT: alpha-1 antitrypsin; AZA: azathioprine; CsA: cyclosporine; CY: cyclophosphamide; MMF: mycophenolate mofetil; MP: methylprednisolone pulse therapy; PD: prednisone; RTX: rituximab; TP: total proteins (normal range: 60–80g/l).
At 24 months, she had her first relapse, presenting with ascites and edema, and no other clinical manifestations of SLE activity. An analysis revealed low C3 levels and, again, treatment consisted of iv pulses of 1g/day of MP administered on 3 consecutive days, as well as iv CY at a dose of 1g/month. She continued with 200mg/day of cyclosporine as maintenance therapy. The latter had to be discontinued because of gastrointestinal intolerance. The patient's symptoms were reactivated after the prednisone dose was reduced to less than 30mg/day. As a complication of the prolonged corticosteroid therapy, she developed avascular osteonecrosis in both knees.
One year later, the patient had a second relapse, with ascites and edema, but no other symptoms of SLE activity. Once again, treatment consisted of 6 doses of 500mg of CY at 2-week intervals. Fifteen months later, she had a new recurrence, with a marked deterioration of her nutritional status; she was taking corticosteroids at a dose of 20mg/day. Parenteral nutrition was started and she underwent treatment with mycophenolate mofetil (MMF) at a dose of 3g/day for 3 months. As there was no improvement in her clinical status or analytical results, her treatment was changed to RTX at a dose of 375mg/m2 weekly for 4 weeks, associated with 2g of MMF, and complete remission was achieved 5 months later.
Given the severity of the disease and its refractoriness to different immunosuppressive agents, the decision was made to administer a new dose of RTX 6 months later. CD19 lymphocytes were not monitored. At the present time, the patient's SLE and PLE have been in remission for 2 years and she continues to take 2mg/day of prednisone and 1g/day of MMF. During the follow-up period, there has been no evidence of any adverse effect related to RTX.
DiscussionProtein-losing enteropathy is a condition associated with the loss of proteins via the gastrointestinal tract. The clinical signs are abdominal pain, diarrhea of varying severity and peripheral edema. Hypoalbuminemia with no evidence of proteinuria is the most common finding.5 It can be associated with a wide variety of disorders such as inflammatory bowel disease, tuberculosis, lymphoma, lymphangiectasia, Whipple's disease, celiac disease, amyloidosis and autoimmune diseases.
Protein-losing enteropathy is an uncommon manifestation in SLE. In a series of 15 SLE patients at the Peking Union Medical College Hospital in Beijing, China, PLE was the presenting sign of SLE in 53%; only 40% complained of diarrhea and abdominal pain. The majority had some degree of peripheral edema: ascites (73%), pleural effusion (60%) and pericardial effusion (47%). All the patients had hypoalbuminemia, and 80% had hypocomplementemia, 67% had dyslipidemia and 40% had hypocalcemia.6 Although the cause is unknown, several pathogenic mechanisms have been proposed: complement-mediated vascular injury, non-necrotizing mesenteric vasculitis, acquired lymphangiectasia and increased permeability of the intestinal microvasculature mediated by interferon gamma, interleukin 6, tumor necrosis factor-α and other cytokines.7–11
The diagnosis of PLE is based on the combination of the clinical signs, demonstration of the loss of proteins into the gastrointestinal tract by means of 99mTc-labeled serum albumin scintigraphy, fecal A1AT clearance, response to treatment and the exclusion of other causes of hypoalbuminemia.299mTc scintigraphy is the diagnostic study of choice over A1AT clearance as it has a higher sensitivity (100% vs 46%) and negative predictive value (100% vs 63%). On the other hand, it makes it possible to trace the origin of the enteric protein loss.12,13 In the case reported here, 99mTc scintigraphy was not performed as it was not available. We used A1AT clearance, which enabled us to follow the patient's course and evaluate the response to treatment.
In a systematic review of 112 patients with PLE associated with SLE, 42% responded to steroid therapy and 66% required another immunosuppressive drug, which included CY in 46%, AZA in 33% and the combination of AZA and CY in 7%.5 With respect to the indication for RTX, to date, only one case of PLE associated with Sjögren's syndrome has been reported, and the response to this monoclonal antibody was good.14
Rutiximab is a monoclonal antibody against the B lymphocyte antigen CD20. Although there are no randomized controlled clinical trials showing the benefits of RTX in SLE, evidence of its efficacy has been shown in observational studies and case reports. At any rate, its use has been indicated for the treatment of patients with refractory forms, defined as individuals who did not achieve remission or had relapsing disease, despite an optimal corticosteroid dose, and the failure of at least 2 immunosuppressive agents. Ramos-Casals et al. analyzed the efficacy of the off-label use of RTX in patients with SLE and found a response of over 80% among patients with proliferative nephritis and neurological and hematological compromise (hemolytic disease and thrombocytopenia); in contrast, the response rate was lower than 50% in those with signs of cutaneous and joint involvement. On the other hand, it made it possible to reduce steroid doses in 79% and discontinue steroid therapy altogether in 14%.15,16
In conclusion, PLE is an uncommon complication of lupus that should be suspected in patients with persistent hypoalbuminemia, peripheral edema, pleural effusion and ascites, once other diseases, mainly kidney and liver disease, have been ruled out. Treatment with RTX should be considered as soon as therapy with a second immunosuppressive agent fails. This approach would probably prevent the development of irreversible damage related to corticosteroid therapy.
Ethical DisclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Sansinanea P, Carrica SA, Marcos J, García MA. Enteropatía perdedora de proteínas asociada a lupus eritematoso sistémico refractaria con buena respuesta a rituximab. Reumatol Clin. 2016;12:47–49.