To analyze the safety of biologic (DMARDs-b) and synthetic targeted therapies (DMARDs-sd) in the BIOBADAGUAY registry (Paraguayan-Uruguayan registry of adverse events (AEs) in patients with inflammatory rheumatic diseases).
MethodsBIOBADAGUAY is a registry to prospectively evaluate the efficacy and safety of FAME-b and FAME-sd. The full methodology is available at https://biobadaguay.ser.es. Variables associated with the safety of the therapies were used for the present study. The incidence of AA was calculated as incidence rate (IR) per 1000 patient-years, with 95% confidence intervals (CI) and Poisson regression for the incidence rate ratio (IRR).
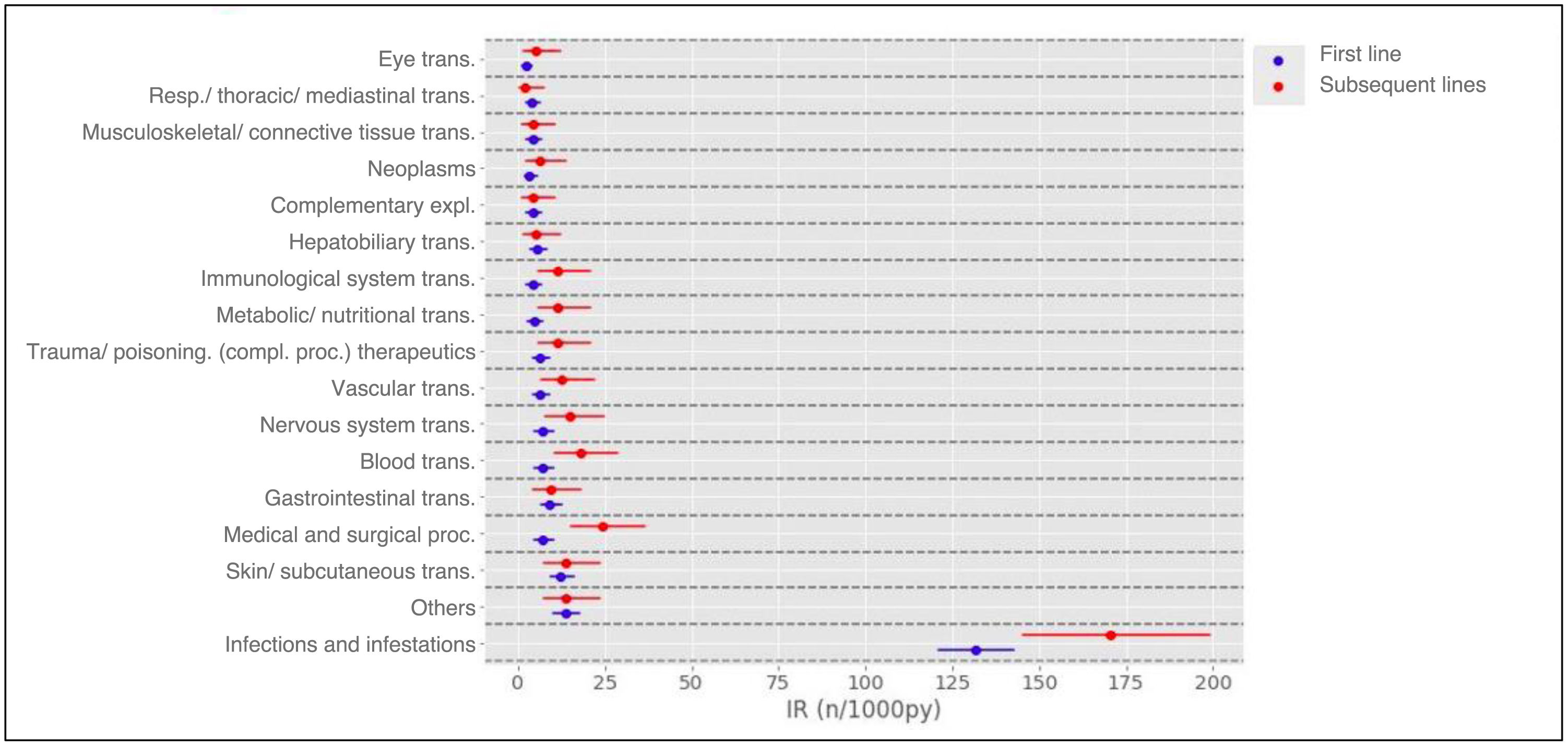
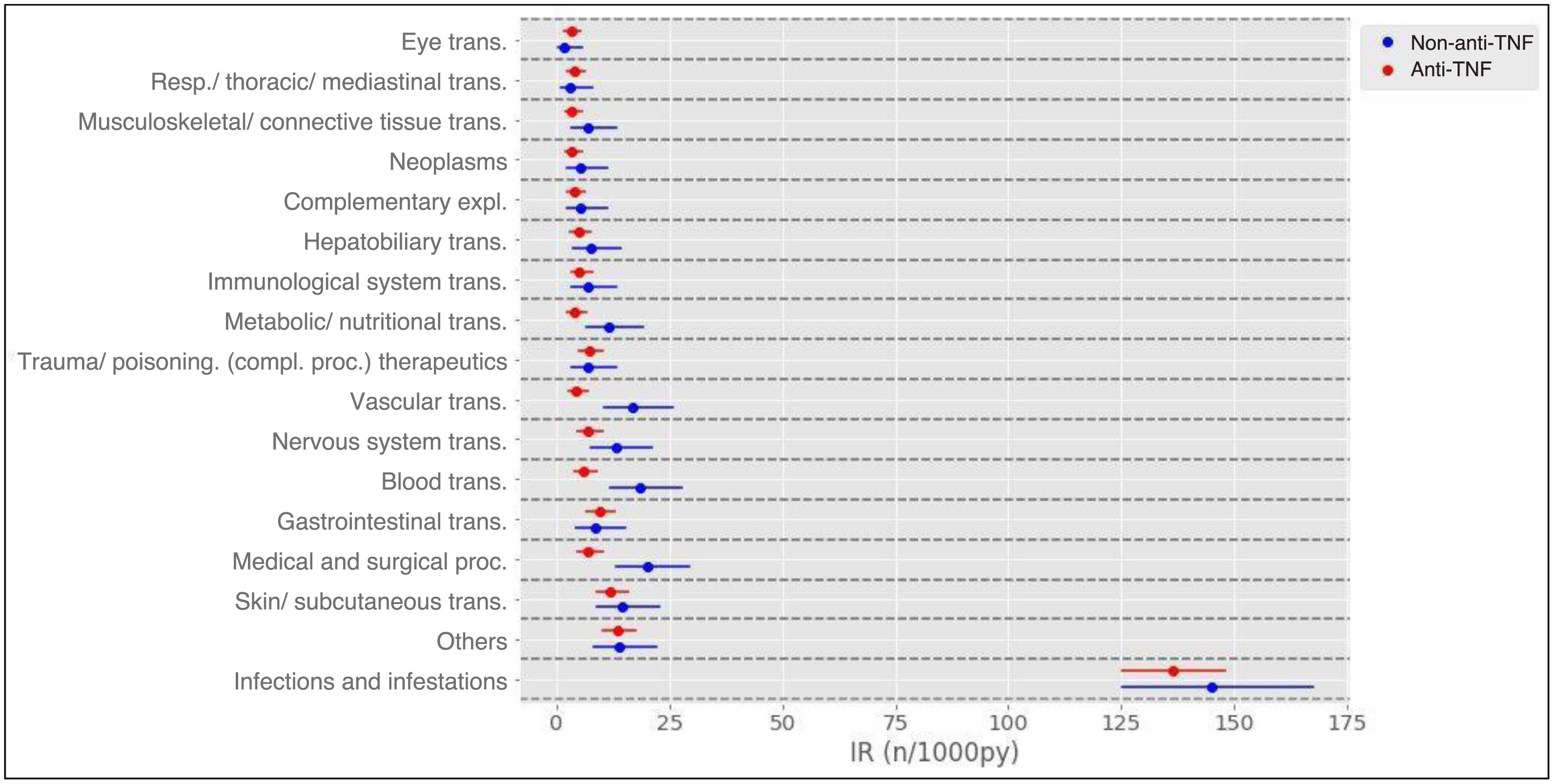
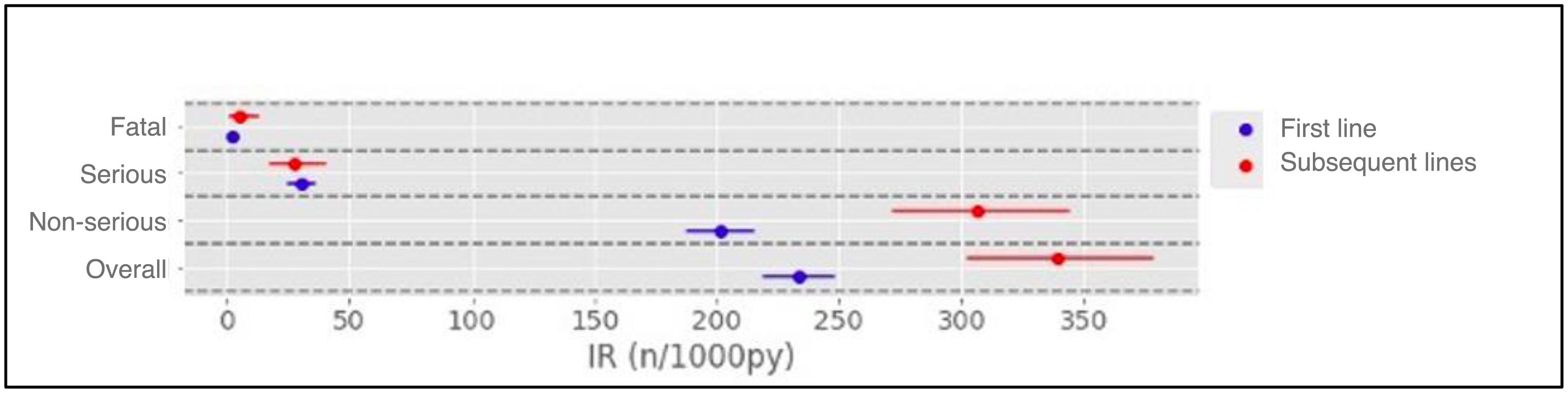
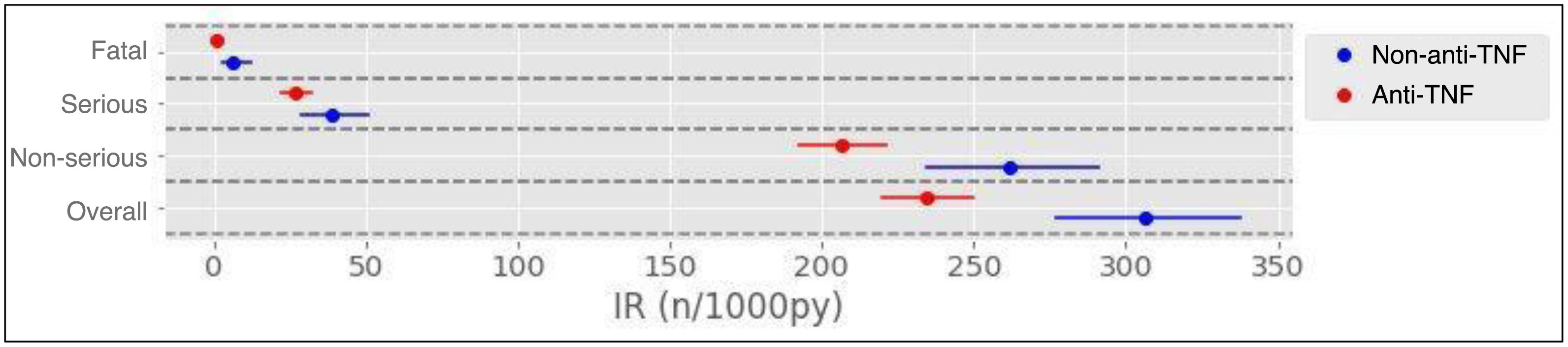
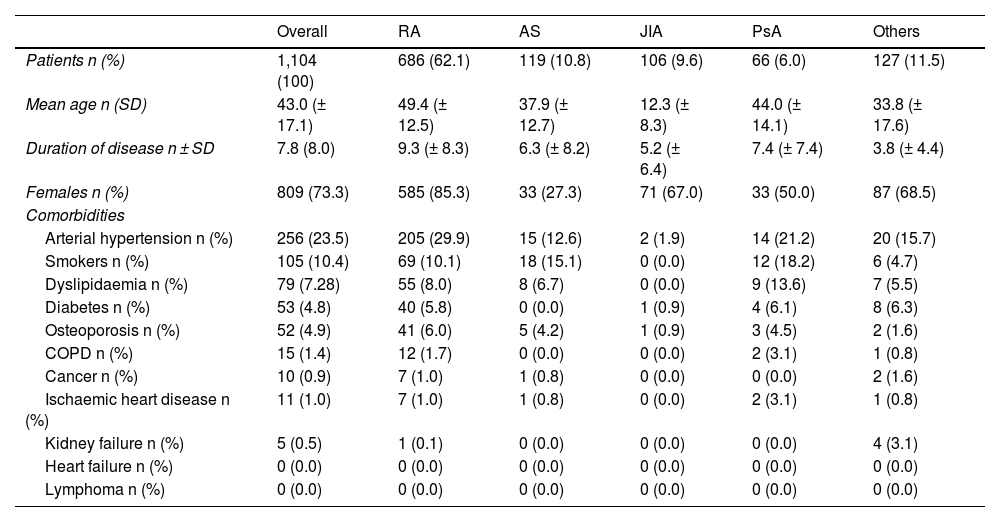
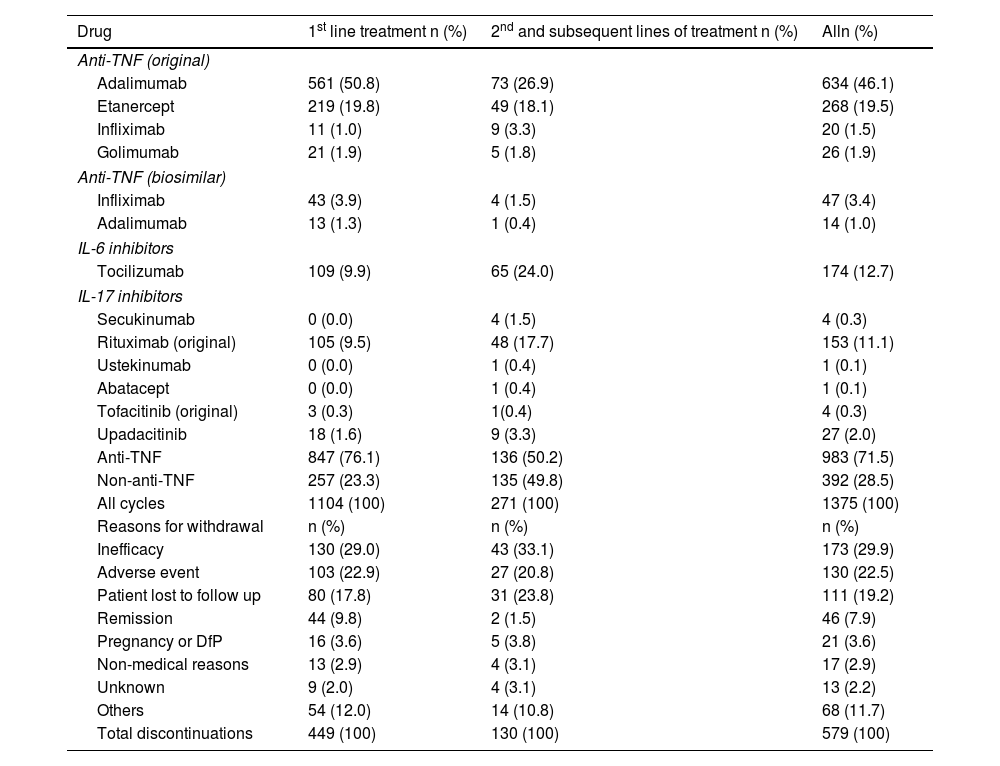
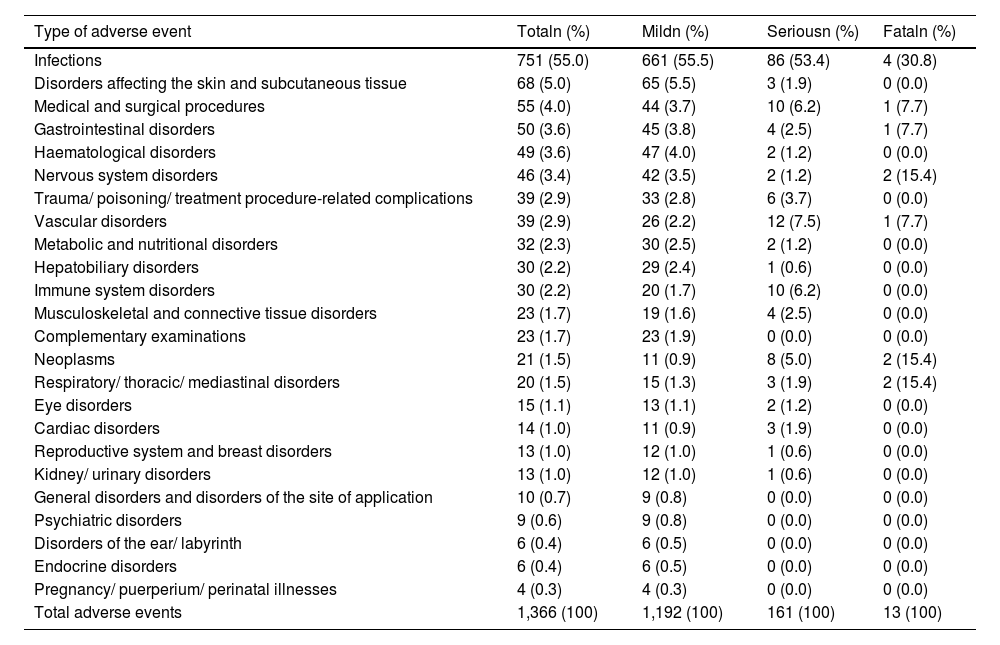
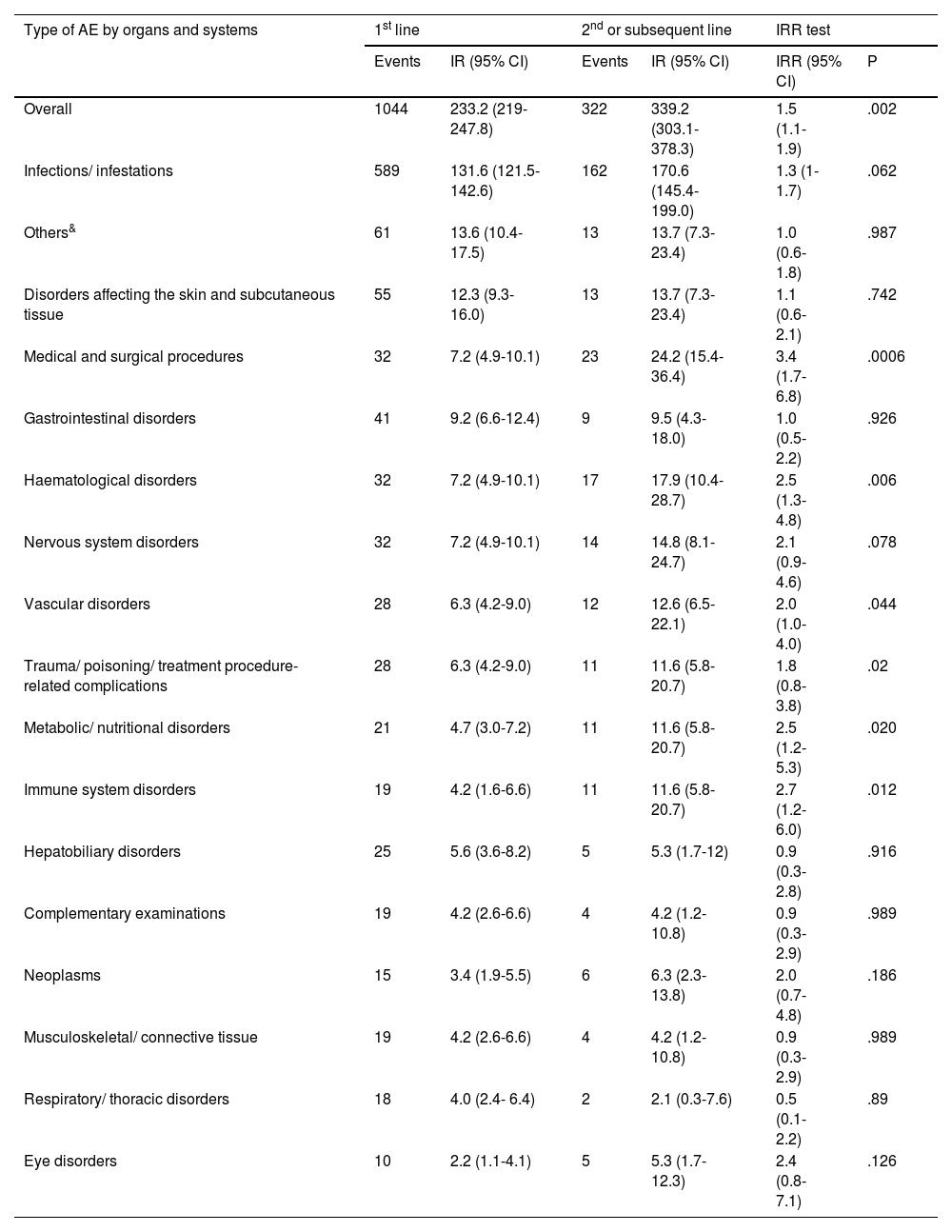
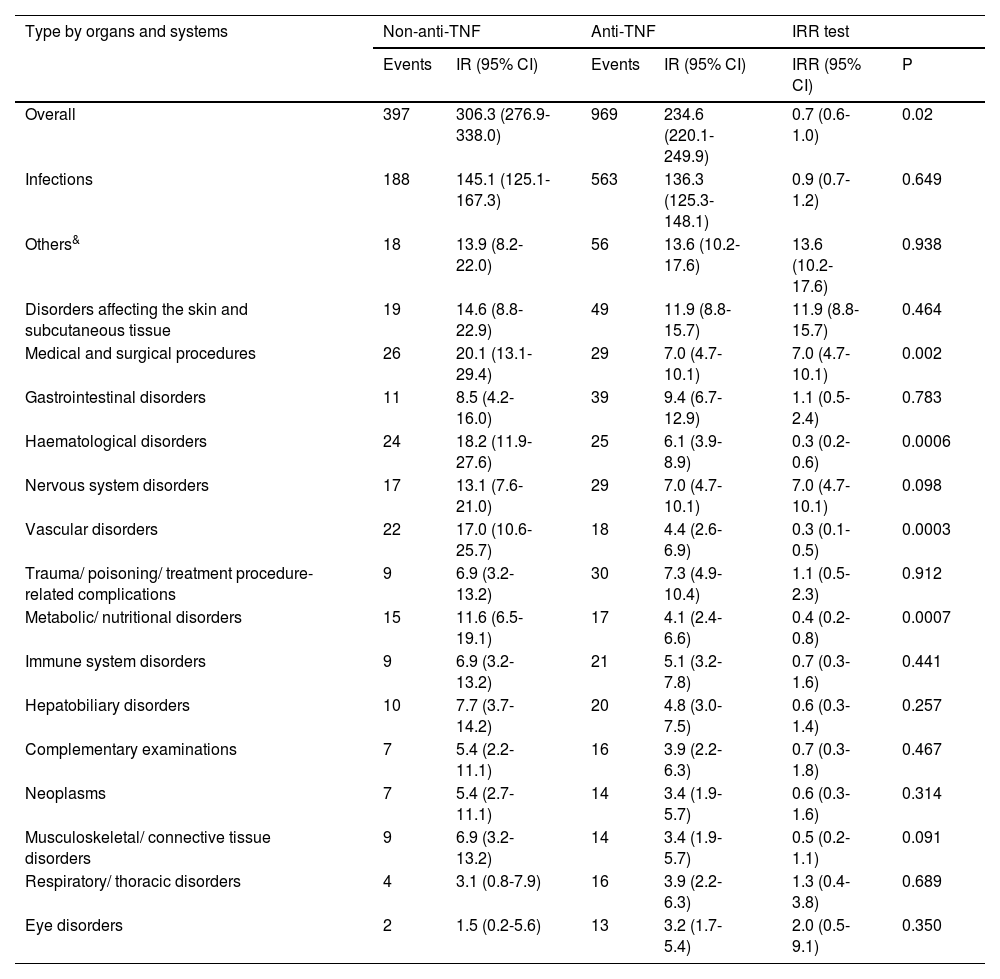
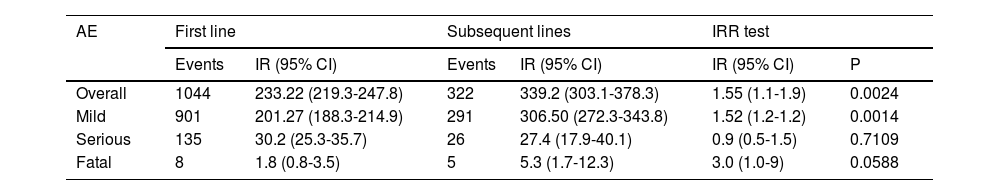
Results1104 patients (73.3% female) with 1366 AA, predominantly mild (87.2%), were analyzed. The overall incidence of AEs was 251.75 per 1000 patient-years. Infections were the most frequent (55.0%), with an incidence of 138.4 per 1000 patient-years. Rheumatoid arthritis and corticosteroid use were associated with more global AEs, while anti-TNF was associated with less AEs.
ConclusionsThis study from the BIOBADAGUAY registry has provided valuable data on the safety of DMARD-b, sd in a cohort of patients with inflammatory rheumatic diseases. The incidence of predominantly mild AEs, with infections as the most frequent adverse event, underscores the need for rigorous and constant monitoring in this population.
Analizar la seguridad de las terapias biológicas (FAME-b) y sintéticas dirigidas (FAME-sd) en el registro BIOBADAGUAY (registro paraguayo-uruguayo de acontecimientos adversos (AA) en pacientes con enfermedades reumáticas inflamatorias).
MétodoBIOBADAGUAY es un registro para evaluar prospectivamente la eficacia y seguridad de los FAME-b y FAME-sd. La metodología completa está disponible en https://biobadaguay.ser.es. Para el presente estudio se utilizaron variables asociadas a la seguridad de las terapias. La incidencia de AA se calculó como tasa de incidencia (IR) por 1000 pacientes-año, con intervalos de confianza (IC) del 95% y la regresión de Poisson para la razón de tasa de incidencia (IRR).
ResultadosSe analizaron 1104 pacientes (73,3% mujeres) con 1366 AA, predominantemente leves (87,2%). La incidencia global de AA fue de 251,75 por 1000 pacientes-año. Las infecciones fueron las más frecuentes (55,0%), con una incidencia de 138,4 por 1000 pacientes-año. La artritis reumatoide y el uso de corticoides se asociaron con más AA globales, mientras que los anti-TNF se asociaron con menos AA.
ConclusionesEste estudio del registro BIOBADAGUAY ha proporcionado datos valiosos sobre la seguridad de los FAME-b, sd en una cohorte de pacientes con enfermedades reumáticas inflamatorias. La incidencia de AA, predominantemente leves y con las infecciones como el evento adverso más frecuente, subraya la necesidad de mantener un monitoreo riguroso y constante en esta población.