The biologics used in the management of rheumatoid arthritis (RA) in recent years have comprehensively permitted to understand their security, as shown in registries such as BIOBADASER. The present manuscript represents an observational cohort study to describe the safety perinfusional profile of those intravenous treatments.
ObjectivesTo confirm the safety profile of biological therapies in routine clinical practice, after the administration of intravenous drugs and 24h post-administration.
Material and methodsWe evaluated a cross-sectional cohort of 114 patients with RA (according to the American College of Rheumatology ACR criteria), attending within one month in 2009 the nursing clinics of day care hospital of 12 Catalonian hospitals. All patients were treated with intravenous biological agents. We recorded the age, sex, current and previous drug treatments. We also collected data about previous vaccination and premedication received and any adverse event occurring at the time of drug administration or within 24h. If an adverse event occurred, it was categorized by MedDRAv11.0 International Dictionary, and categorized in terms of intensity (mild, moderate, severe), relationship to drug administration according to Karch and Lasagna algorithm (unrelated, unlikely, possible, probable, definite) and the further measures taken.
Results111 patients met the inclusion criteria, with a mean age of 56.06 years (SD: 12.12), 90 of them women (81.1%) and mean time since diagnosis of the disease of 11.97 years (SD: 7.95). 24 patients (21.6%) had a history of allergy. 12 adverse events were observed in 7 patients, 9 of which at the time of administration and 3 in 24h after. There were no serious adverse events and only one of the adverse events (AEs) was rated as moderate (urticaria). The remaining AEs were mild.
El uso de biológicos ha permitido conocer de manera exhaustiva su seguridad gracias a registros como BIOBADASER. El presente trabajo permite, con un estudio observacional de cohortes, describir el perfil de seguridad perinfusional de dichos tratamientos por vía intravenosa.
ObjetivosConocer el perfil de seguridad en la práctica clínica, tras la administración de biológicos por vía intravenosa y durante las 24h posteriores.
Material y métodosCohorte transversal de 114 pacientes con AR tratados con agentes biológicos (criterios ACR) durante un mes de 2009 por enfermería de hospital de día de 12 centros hospitalarios catalanes. Se analizaron la edad, el sexo, los tratamientos actuales y previos, los datos de vacunación previa y la premedicación. Se registró también cualquier acontecimiento adverso (AA) durante la administración o en las 24h posteriores. Se clasificó según el diccionario internacional MedDRAv11.0 y se describieron la intensidad (leve, moderada, severa), la relación con la administración del fármaco según el algoritmo de Karch y Lasagna (no relacionada, improbable, posible, probable, definitiva) y las medidas emprendidas. El análisis estadístico se realizó mediante SPSS 18.0.
ResultadosCiento once con criterios de inclusión (edad media ± desviación estándar 56,06 ± 12,12 años), 90 mujeres (81,1%) y evolución de 11,97 ± 7.95 años; 24 pacientes (21,6%) con antecedentes de alergia. Se observaron 12 AA en 7 pacientes, 9 de ellos durante la administración y 3 en las 24h posteriores. No hubo ningún acontecimiento adverso grave y uno de los AA se calificó de intensidad moderada (urticaria). El resto de los AA fueron de intensidad leve.
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that causes progressive destruction and swelling, pain, morning stiffness and loss of functional capacity, mainly affecting the hands and feet. The prevalence in our country is 0.5%, representing about 200000 adults affected over 20 years.1,2 The loss of function of the joints decreases the ability to perform activities of daily living, causes problems in the workplace and limits considerably the quality of life related to health. RA-related mortality is higher than the general population and a decrease in life expectancy between 5 and 10 years is seen.3,4 The costs of the disease are a huge consumer of health and social resources, estimated annually at 1.120 million euros for these 200000 patients.4,5
The current goal of RA treatment is to induce remission or, failing that, to achieve a low disease inflammatory activity. Early and active treatment in the initial stages of the disease is essential for good long term prognosis.5,6 Among existing treatments, biological therapies have represented a breakthrough in the treatment of RA.
The role of nursing in the rheumatology day hospital is managing and controlling biological therapy, early detection of side effects, monitoring and reporting patient comorbidity or warning signs to watch in relation to treatment and instruction for patient self-administration of subcutaneous biologics.7,8 The biologic drugs most commonly administered intravenously in the day hospital are infliximab (Remicade®), rituximab (Mabthera®), abatacept (Orencia®) and tocilizumab (RoActemra®).
This study evaluates the safety profile of biologic therapies in routine clinical practice conditions, after administration of intravenous drugs and for 24h after their administration, and to describe the role of nursing in the whole disease and treatment process.
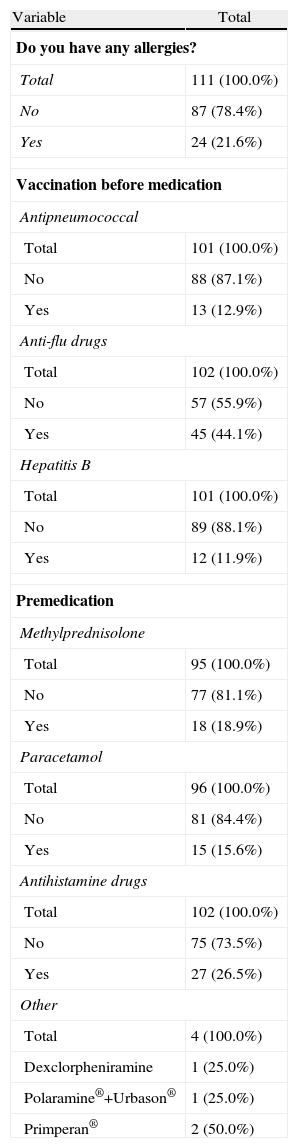
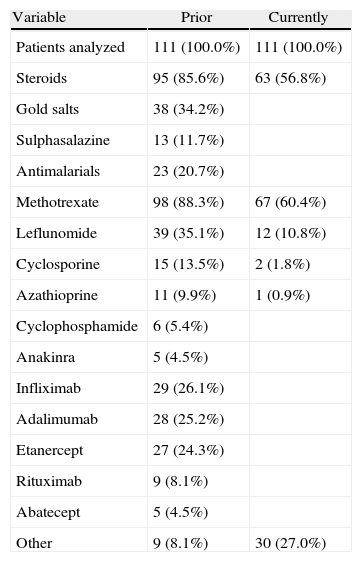
Materials and MethodsOf the 114 patients enrolled, we analyzed a cross-sectional cohort of 111 patients, with a mean age±SD of 56.06±12.1 years, 90 (81.1%) women, 21 (18.9%) men, and a time since onset of the disease of 11.97±7.95 years. One hundred and eleven patients fulfilled the criteria for RA according to American College of Rheumatology (ACR), and were evaluated during the period between November 15 and December 15, 2009 in day hospitals and nursing visits at 12 hospitals in Catalonia. All patients gave written informed consent to participate in the study. Demographic variables were recorded in a notebook: age and sex of the patients, the current and previous drug treatments and vaccination data as well as prior premedication (Tables 1 and 2). We noted any adverse events (AEs) that occurred at the time of drug administration or after 24h. The AEs recorded were classified according to the International MedDRAv11.0 Dictionary, describing the degree of intensity (mild, moderate, severe) and were assessed regarding drug administration by the Karch and Lasagna algorithm (unrelated, unlikely, possible, likely, definite) and the action taken. The procedures performed before, during and after drug administration by nurses were recorded to monitor the process. They made an assessment and hemodynamic monitoring, punctured a peripheral vein, which after extraction of a control blood sample, was used for intravenous treatment with a continuous infusion pump according to protocol. Drug administration was conducted by infusion pump intravenously in 88 patients (80%–0%) and without a pump in 22 (20%) patients, with a mean of 18.3 infusions and a median of 9.5.
Clinical Parameters: Allergies, Vaccination and Premedication.
| Variable | Total |
| Do you have any allergies? | |
| Total | 111 (100.0%) |
| No | 87 (78.4%) |
| Yes | 24 (21.6%) |
| Vaccination before medication | |
| Antipneumococcal | |
| Total | 101 (100.0%) |
| No | 88 (87.1%) |
| Yes | 13 (12.9%) |
| Anti-flu drugs | |
| Total | 102 (100.0%) |
| No | 57 (55.9%) |
| Yes | 45 (44.1%) |
| Hepatitis B | |
| Total | 101 (100.0%) |
| No | 89 (88.1%) |
| Yes | 12 (11.9%) |
| Premedication | |
| Methylprednisolone | |
| Total | 95 (100.0%) |
| No | 77 (81.1%) |
| Yes | 18 (18.9%) |
| Paracetamol | |
| Total | 96 (100.0%) |
| No | 81 (84.4%) |
| Yes | 15 (15.6%) |
| Antihistamine drugs | |
| Total | 102 (100.0%) |
| No | 75 (73.5%) |
| Yes | 27 (26.5%) |
| Other | |
| Total | 4 (100.0%) |
| Dexclorpheniramine | 1 (25.0%) |
| Polaramine®+Urbason® | 1 (25.0%) |
| Primperan® | 2 (50.0%) |
Prior and Current Treatments for RA.
| Variable | Prior | Currently |
| Patients analyzed | 111 (100.0%) | 111 (100.0%) |
| Steroids | 95 (85.6%) | 63 (56.8%) |
| Gold salts | 38 (34.2%) | |
| Sulphasalazine | 13 (11.7%) | |
| Antimalarials | 23 (20.7%) | |
| Methotrexate | 98 (88.3%) | 67 (60.4%) |
| Leflunomide | 39 (35.1%) | 12 (10.8%) |
| Cyclosporine | 15 (13.5%) | 2 (1.8%) |
| Azathioprine | 11 (9.9%) | 1 (0.9%) |
| Cyclophosphamide | 6 (5.4%) | |
| Anakinra | 5 (4.5%) | |
| Infliximab | 29 (26.1%) | |
| Adalimumab | 28 (25.2%) | |
| Etanercept | 27 (24.3%) | |
| Rituximab | 9 (8.1%) | |
| Abatecept | 5 (4.5%) | |
| Other | 9 (8.1%) | 30 (27.0%) |
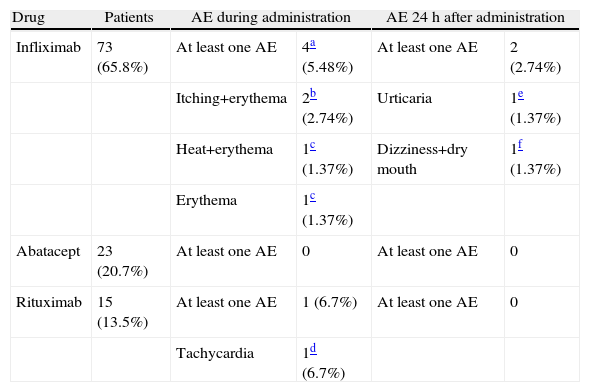
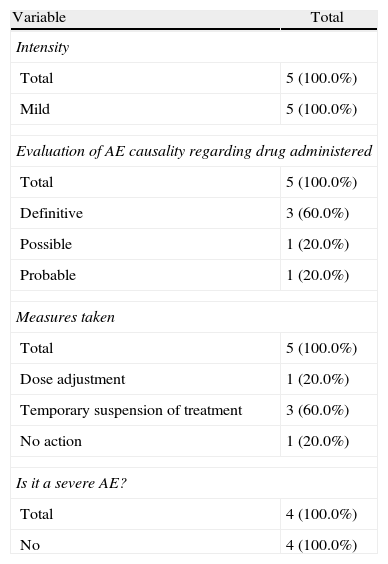
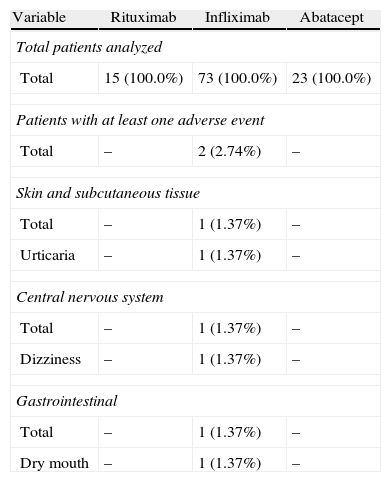
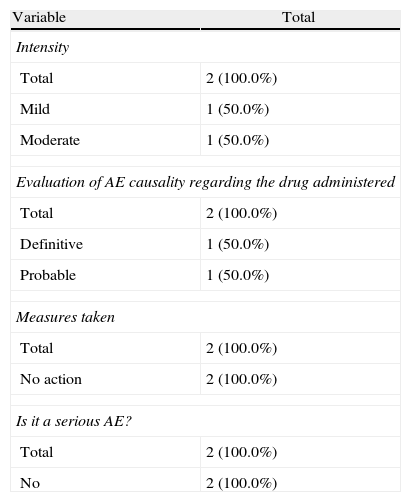
During the administration and after 24h, in the 111 patients who met the inclusion criteria, we found 24 patients (21.6%) who had a history of allergy. There were a total of 12 AEs in 7 patients, 9 of which occurred at the time of administration and 3 in the subsequent 24h (Table 3). There were no severe AEs and only one moderate AE (urticaria). The remaining AEs were mild. The reaction characteristics during and after infusion, and drugs involved are presented in Tables 4–7.
Adverse Events During Administration and 24h After Administration: Relation to Drug and Measures Taken.
| Drug | Patients | AE during administration | AE 24h after administration | ||
| Infliximab | 73 (65.8%) | At least one AE | 4a (5.48%) | At least one AE | 2 (2.74%) |
| Itching+erythema | 2b (2.74%) | Urticaria | 1e (1.37%) | ||
| Heat+erythema | 1c (1.37%) | Dizziness+dry mouth | 1f (1.37%) | ||
| Erythema | 1c (1.37%) | ||||
| Abatacept | 23 (20.7%) | At least one AE | 0 | At least one AE | 0 |
| Rituximab | 15 (13.5%) | At least one AE | 1 (6.7%) | At least one AE | 0 |
| Tachycardia | 1d (6.7%) | ||||
The management of adverse events (AE) was done during clinical and hemodynamic patient monitoring, until resolution of the acute episode. No treatment for severe AE was employed.
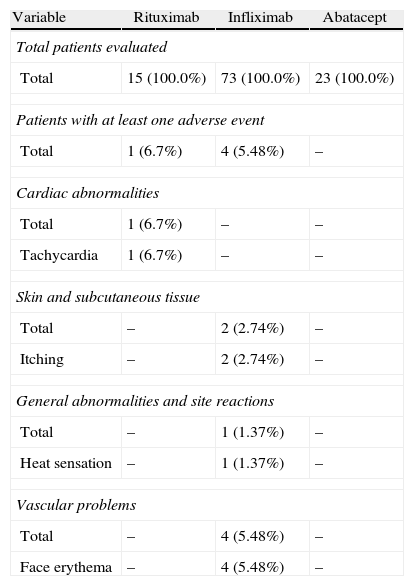
Adverse Events During Current Drug Treatment.
| Variable | Rituximab | Infliximab | Abatacept |
| Total patients evaluated | |||
| Total | 15 (100.0%) | 73 (100.0%) | 23 (100.0%) |
| Patients with at least one adverse event | |||
| Total | 1 (6.7%) | 4 (5.48%) | – |
| Cardiac abnormalities | |||
| Total | 1 (6.7%) | – | – |
| Tachycardia | 1 (6.7%) | – | – |
| Skin and subcutaneous tissue | |||
| Total | – | 2 (2.74%) | – |
| Itching | – | 2 (2.74%) | – |
| General abnormalities and site reactions | |||
| Total | – | 1 (1.37%) | – |
| Heat sensation | – | 1 (1.37%) | – |
| Vascular problems | |||
| Total | – | 4 (5.48%) | – |
| Face erythema | – | 4 (5.48%) | – |
Characteristics of Adverse Events During Treatment Administration.
| Variable | Total |
| Intensity | |
| Total | 5 (100.0%) |
| Mild | 5 (100.0%) |
| Evaluation of AE causality regarding drug administered | |
| Total | 5 (100.0%) |
| Definitive | 3 (60.0%) |
| Possible | 1 (20.0%) |
| Probable | 1 (20.0%) |
| Measures taken | |
| Total | 5 (100.0%) |
| Dose adjustment | 1 (20.0%) |
| Temporary suspension of treatment | 3 (60.0%) |
| No action | 1 (20.0%) |
| Is it a severe AE? | |
| Total | 4 (100.0%) |
| No | 4 (100.0%) |
Adverse Events 24h After Treatment Drug Administration.
| Variable | Rituximab | Infliximab | Abatacept |
| Total patients analyzed | |||
| Total | 15 (100.0%) | 73 (100.0%) | 23 (100.0%) |
| Patients with at least one adverse event | |||
| Total | – | 2 (2.74%) | – |
| Skin and subcutaneous tissue | |||
| Total | – | 1 (1.37%) | – |
| Urticaria | – | 1 (1.37%) | – |
| Central nervous system | |||
| Total | – | 1 (1.37%) | – |
| Dizziness | – | 1 (1.37%) | – |
| Gastrointestinal | |||
| Total | – | 1 (1.37%) | – |
| Dry mouth | – | 1 (1.37%) | – |
Characteristics of the Adverse Events in the 24h After Drug Administration.
| Variable | Total |
| Intensity | |
| Total | 2 (100.0%) |
| Mild | 1 (50.0%) |
| Moderate | 1 (50.0%) |
| Evaluation of AE causality regarding the drug administered | |
| Total | 2 (100.0%) |
| Definitive | 1 (50.0%) |
| Probable | 1 (50.0%) |
| Measures taken | |
| Total | 2 (100.0%) |
| No action | 2 (100.0%) |
| Is it a serious AE? | |
| Total | 2 (100.0%) |
| No | 2 (100.0%) |
Intravenous biological therapies infliximab, rituximab, abatacept and tocilizumab are effective in the treatment of RA and other chronic inflammatory diseases that present with outbreaks. Infliximab, an anti-TNF-α, is administered through intravenous administration at a frequency of every 8 weeks, and is today the most widely used and the most common in the day hospital. Rituximab is a chimeric monoclonal antibody that acts on B lymphocytes, its administration is carried out in 2 cycles per year, approximately every 6 months, and its handling protocolized at the day hospital, and its administration requires premedication. Less frequently, abatacept, which is a protein obtained by cell culture that blocks T cell costimulation, is also administered intravenously monthly. Among the complications that occur more often in this type of therapy, most are related to administration. These reactions can be classified into acute or delayed, present at the time of administration or within 24h (acute) or those occurring from 24h to 14 days after infusion (late).
In this study we focused on the acute AE, occurring during drug administration or within 24h, in RA patients in clinical practice conditions. AEs observed were acute pruritus, edema, urticaria, hypotension, bradycardia, tachycardia, headache, fever or anaphylactic reactions, among others.
The BIOBADASER registry (Adverse Event Registry for Biologic Therapies), created in 2001 to determine the safety of biological therapies in rheumatic diseases, provides safety data and relevant AE retention5 rates. Comparatively, a total of 12 AEs were observed in 7 patients, included in the 111 patients, 9 of which occurred at the time of administration and 3 in after 24h. These data correspond to 10.8% of patients with AE, 8.1% for administration and 2.7% in the next 24h. BIOBADASER5 has reported a total of 761 reactions associated with treatment, taking into account that, in November 2009, 5493 patients had been recorded, corresponding to a rate of 13.8% of patients with treatment-associated AE. If we compare these results with those in BIOBADASER, it appears that although the present sample is smaller, the percentage of AE is no different from BIOBADASER. Keep in mind that the percentage of BIOBADASER also includes those AEs observed in subcutaneously administered drugs, while in a study conducted by the BIOBADASER group in 2008, it was shown that the frequency of adverse reactions was greatest with infliximab than with other anti-TNF-α by subcutaneous administration. Similarly, in studies with abatacept10 and rituximab,9 the treatment-related AE range from 10% to 15% of patients.
Regarding the frequency of drug-related AEs administered in our sample, we observed that it was higher for infliximab with respect to the other treatments, explained by the size of the sample receiving this treatment (73% of patients) compared to 20, 7% receiving abatacept and 13.5% receiving rituximab. Two patients had AEs during rituximab administration and none of those treated with abatacept had AE. None of the registered AE was severe; only one was rated as moderate intensity (urticaria), and the rest, mild. In most of the published articles, the percentage of severe AE is also low, but intravenously administered drug reactions are, in some cases, the cause of discontinuation of treatment, although in some cases they may be controlled with medication to reduce sensitization or decrease the rate of infusion.
In our cases we established one definite relationship of AE with medication, but in 2 cases we established a probable relationship and in the remaining case the established relationship was possible. Three of the cases were resolved with temporary treatment interruption, in one case the dose was adjusted and the remaining 3 did not require any action. Regarding the appearance of AE during treatment delivery or within the following 24h, we note that in 71.5% of patients the reaction occurred during treatment and in 28.5% it occurred in the following 24h.
This paper aims to highlight key data in the management with biological therapies. First, it should be noted that the type of treatment-related reactions observed in intravenous administration drugs is similar to that described above in different studies using biological treatments. Second, we have not observed any serious AE, although it is important to be alert to the possible occurrence of these AEs during and 24h after infusion, which may be a cause for discontinuation. It also emphasizes the high prevalence of corticosteroid treatment of RA patients, something not always recorded, and the low rate of influenza and pneumococcal vaccination, despite existing recommendations. Finally, it should be noted that monitoring should be done not only during infusion administration, but nursing plays a role in the subsequent control in the 24h after administration.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of InterestThe present multicentric study was done in collaboration between all of the authors. Roche Pharma collaborated unrestrictedly in data capture and logistics. It has not participated in the elaboration of the document. There was no financing for the project.
Please, cite this article as: Corominas H, et al. Perfil de seguridad de las terapias biológicas intravenosas en una cohorte de pacientes con artritis reumatoide. Monitorización clínica por enfermería (estudio Sebiol). Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2012.06.001.