Shrinking lung syndrome (SLS) and pleural effusion (PE) are rare manifestations of primary Sjögren's syndrome (pSS).1,2 We present the case of a female patient with these manifestations of the onset of pSS.
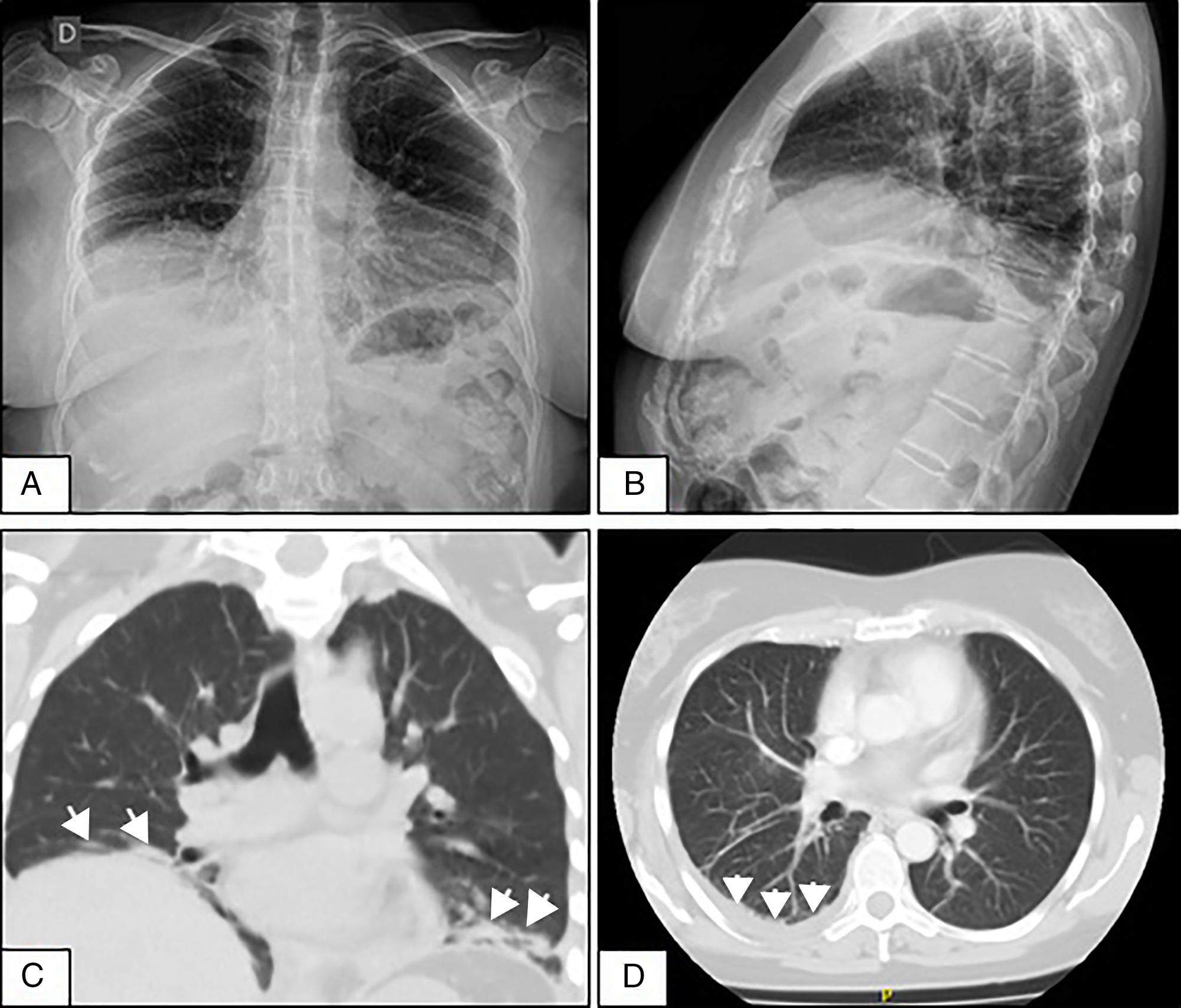
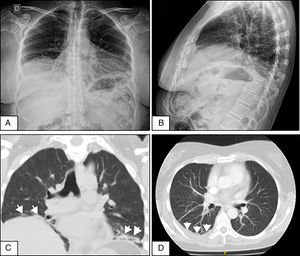
A 50-year-old woman who consulted with a 4-week history of dyspnoea on medium exertion, right-sided pleuritic pain and constitutional symptoms. Two weeks prior to admission she underwent a chest X-ray showing mild PE, and infectious and tumour processes were ruled out. Due to the persistent symptoms a repeat chest X-ray was performed which showed elevation of both hemidiaphragms and bibasilar laminar atelectasis (Fig. 1A and B). CT scan and chest angiotomography confirmed atelectasis, right-sided PE, with no infiltrates or pulmonary emboli (Fig. 1C and D). Reduced bilateral diaphragmatic excursion was observed on fluoroscopy (sniff test). Thoracentesis was performed; the fluid was compatible with exudate. Severe restriction and reduced maximum inspiratory and expiratory pressures were observed. There was reduced carbon monoxide diffusion capacity. The six-minute walking test showed a 5-point drop in oxygen saturation after 2min. The patient initially denied the presence of sicca symptoms. Mild leukocytosis was observed, elevated erythrocyte sedimentation rate, polyclonal hypergammaglobulinaemia, and viral serologies were negative. Prednisone 20mg/day was started until the immunological results were available. ANA-Hep2, anti-ENA, anti-Ro/SS-A, anti-La/SS-B, anti-ADN and cryoglobulins were negative, normal complement levels, and low-titre positive rheumatoid factor. A month later she manifested xerostomia and xerophthalmia that persisted for more than 3 months. She met 4 out of 6 of the 2002 American-European classification criteria for pSS (oral and eye symptoms, Schirmer test less than 5mm in 5min, van Bijsterveld rose bengal staining score of 6, and minor salivary gland biopsy with Chisholm and Mason grade 4 lymphocytic sialadenitis).3 SLS in pSS was diagnosed with an activity rate (ESSDAI)4 of 5. As the patient progressed, she presented lymphocytic vasculitis of the skin, and axonal sensitivomotor polyneuropathy. She completed treatment with azathioprine 150mg/day and prednisone 40mg/day, with incomplete response and developed pancytopenia. She was switched to mycophenolate mofetil with haematological and cutaneous response, but neurological and lung deterioration (ESSDAI 15). Two doses of 1g rituximab were administered every 15 days, and inhaled budesonide. After one month the ESSDAI was 7, and at 3 months the respiratory symptoms had improved, the walking test normalised, moderate restriction on spirometry, absence of pleural effusion, but the diaphragmatic elevation persisted. The Sjögren's Syndrome Disease Damage Index5 was 7.
(A and B) Front and side-view chest X-rays showing the elevation of both hemidiaphragms and bibasilar laminar atelectasis. (C) Coronal CT without contrast material showing diaphragmatic elevation and subsegmental atelectasis (arrows) in both lung bases. (D) Axial CT of the chest with contrast material showing slight pleural effusion in the right lung base (arrows).
Extraglandular manifestations can appear in up to 50% of patients with pSS, and only 11% will suffer lung impairment.4 Tracheitis, bronchitis and bronchiolitis, together with interstitiopathies (non-specific interstitial pneumonia, usual interstitial pneumonia, lymphocytic interstitial pneumonia) are the most frequent manifestations.4,6
SLS is an exceptional manifestation in pSS.1,7 Two previous cases have been reported in the literature (Table 1).1,8 It is characterised by reduced lung volumes, diaphragmatic elevation, restrictive physiology, and no parenchymal involvement.1,7–9 It usually presents with dyspnoea on exertion, orthopnoea, and/or pleuritic pain.8,9 Various pathogenic mechanisms have been postulated to explain the diaphragmatic dysfunction: myositis, fibrosis, phrenic neuropathy and/or intercostal neuropathy, or a combination.8,9 Fluoroscopy with sniff test and ultrasound can reveal dynamic impairment of diaphragmatic function. Chest X-ray and CT can reveal reduced lung volumes and atelectasis.1,8–11 Low mortality attributable to SLS has been reported, but it causes high morbidity, and there is functional improvement in only 20%.10 There is no standard treatment because of its low prevalence.1,7–11 Good response has been reported to prednisone 30–40mg/day, in combination with azathioprine or cyclophosphamide in severe cases.7–11 There are publications that report improvement with rituximab in 2 patients with lupus, and one patient with pSS.1,7–9 There are reports of a slight benefit with β2-agonists and theophylline.9
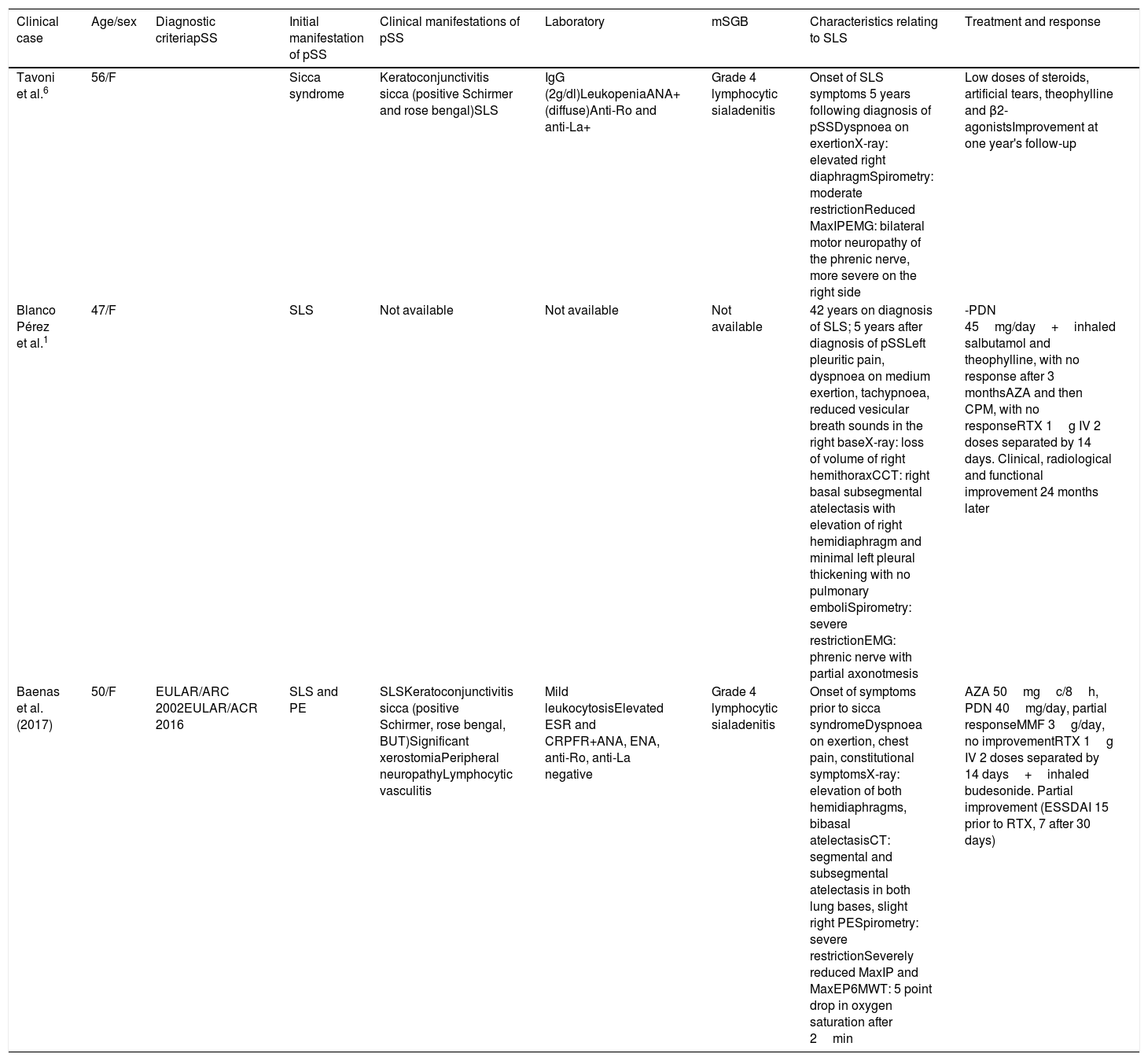
Clinical, functional and imaging characteristics of patients with shrinking lung syndrome associated with Sjögren's syndrome.
| Clinical case | Age/sex | Diagnostic criteriapSS | Initial manifestation of pSS | Clinical manifestations of pSS | Laboratory | mSGB | Characteristics relating to SLS | Treatment and response |
|---|---|---|---|---|---|---|---|---|
| Tavoni et al.6 | 56/F | Sicca syndrome | Keratoconjunctivitis sicca (positive Schirmer and rose bengal)SLS | IgG (2g/dl)LeukopeniaANA+ (diffuse)Anti-Ro and anti-La+ | Grade 4 lymphocytic sialadenitis | Onset of SLS symptoms 5 years following diagnosis of pSSDyspnoea on exertionX-ray: elevated right diaphragmSpirometry: moderate restrictionReduced MaxIPEMG: bilateral motor neuropathy of the phrenic nerve, more severe on the right side | Low doses of steroids, artificial tears, theophylline and β2-agonistsImprovement at one year's follow-up | |
| Blanco Pérez et al.1 | 47/F | SLS | Not available | Not available | Not available | 42 years on diagnosis of SLS; 5 years after diagnosis of pSSLeft pleuritic pain, dyspnoea on medium exertion, tachypnoea, reduced vesicular breath sounds in the right baseX-ray: loss of volume of right hemithoraxCCT: right basal subsegmental atelectasis with elevation of right hemidiaphragm and minimal left pleural thickening with no pulmonary emboliSpirometry: severe restrictionEMG: phrenic nerve with partial axonotmesis | -PDN 45mg/day+inhaled salbutamol and theophylline, with no response after 3 monthsAZA and then CPM, with no responseRTX 1g IV 2 doses separated by 14 days. Clinical, radiological and functional improvement 24 months later | |
| Baenas et al. (2017) | 50/F | EULAR/ARC 2002EULAR/ACR 2016 | SLS and PE | SLSKeratoconjunctivitis sicca (positive Schirmer, rose bengal, BUT)Significant xerostomiaPeripheral neuropathyLymphocytic vasculitis | Mild leukocytosisElevated ESR and CRPFR+ANA, ENA, anti-Ro, anti-La negative | Grade 4 lymphocytic sialadenitis | Onset of symptoms prior to sicca syndromeDyspnoea on exertion, chest pain, constitutional symptomsX-ray: elevation of both hemidiaphragms, bibasal atelectasisCT: segmental and subsegmental atelectasis in both lung bases, slight right PESpirometry: severe restrictionSeverely reduced MaxIP and MaxEP6MWT: 5 point drop in oxygen saturation after 2min | AZA 50mgc/8h, PDN 40mg/day, partial responseMMF 3g/day, no improvementRTX 1g IV 2 doses separated by 14 days+inhaled budesonide. Partial improvement (ESSDAI 15 prior to RTX, 7 after 30 days) |
ANA: antinuclear antibodies; AZA: azathioprine; BUT: break up time; mSGB: minor salivary gland biopsy; CPM: cyclophosphamide; PE: pleural effusion; EMG: electromyogram; F: female; RF: rheumatoid factor; IgG: immunoglobulin G; MMF: mycophenolate mofetil; 6MWT: 6-minute walking test; PDN: prednisone; MaxEP: maximum expiratory pressure; MaxIP: maximum inspiratory pressure; RTX: rituximab; SLS: shrinking lung syndrome; pSS: primary Sjögren's syndrome; CCT: chest CT; ECR: erythrocyte sedimentation rate.
PE is also rare in Sjögren's syndrome (1%–5%, 7%), and is generally associated with another autoimmune disease or heart failure.2 PE associated with pSS can be uni- or bilateral, with fewer than 11,000 cells, and mononuclear predominant exudate. It was striking that in our patient we observed polymorphonuclear predominant exudate. Although PE can be associated with SLS our patient had PE prior to the SLS, therefore we associated this finding with her autoimmune disease. PE associated with pSS generally improves with low doses of glucocorticoids.2
Although SLS and PE are rare manifestations in pSS, they should be considered among the extra-glandular manifestations of the disease. This case also illustrates the possibility of pSS presenting with systemic manifestations before the onset of the typical sicca syndrome symptoms.
Please cite this article as: Baenas DF, Retamozo S, Pirola JP, Caeiro F. Síndrome de pulmón encogido y derrame pleural como manifestación inicial de síndrome de Sjögren primario. Reumatol Clin. 2020;16:65–68.