The SUMAR project established a definition for remission that includes the patient's perspective (global assessment of pain and HAQ) in the definition of remission in rheumatoid arthritis (RA). This new definition needs to be assessed on clinical practice. The main objective of the RA-Remission study is to estimate the proportion of patients in sustained remission according to SUMAR definition through 2-year follow-up, among patients in SUMAR remission at baseline. This manuscript describes the objectives and methodology of the study.
Materials and methodsThis is a longitudinal, prospective, observational and multicentre study, involving 10 centres. Patients with RA who have achieved clinical remission in accordance to their attending rheumatologist clinical judgement within the last 6 months will be included. Follow-up visits will take place at 3, 6, 12, 12, 18 and 24 months. Ultrasound assessment of the power Doppler (PD) signal for 30 joints and 6 tendon regions will be performed at each participating centre by an expert blinded to all other study findings. Definitions of remission according to SUMAR, PD and activity indices will be used. Sustained remission will be considered for each of the definitions when the patient in remission at the baseline visit maintains it during all follow-up visits. To estimate the proportion of patients maintaining remission at two years with an accuracy of at least 6%, 180 patients will need to be recruited.
Discussion and conclusionsThe main strength of the design is the use of ultrasound assessment as the gold standard for assessing inflammatory activity.
El proyecto SUMAR estableció una definición de remisión que incorpora la perspectiva del paciente (la valoración global del dolor y el HAQ) en la definición de la remisión en artritis reumatoide (AR). Esta nueva definición debe ser evaluada en práctica clínica. El objetivo principal del estudio Remisión-AR es estimar durante un seguimiento de 2 años la proporción de pacientes en remisión sostenida según la definición SUMAR. Este manuscrito detalla los objetivos y metodología del estudio.
Materiales y métodosSe trata de un estudio longitudinal, prospectivo, observacional y multicéntrico, con la participación de 10 centros. Se incluirán pacientes con AR que hayan alcanzado remisión clínica en los últimos 6 meses según juicio clínico de su reumatólogo. Se realizarán visitas de seguimiento a los 3, 6, 12, 18 y 24 meses. En cada centro participante, un reumatólogo experto en ecografía, ciego al resto de hallazgos del estudio, valorará la señal Power Doppler (PD) para 30 articulaciones y 6 regiones tendinosas. Se emplearán las definiciones de remisión según SUMAR, PD e índices de actividad. Se considerará remisión sostenida para cada una de las definiciones, cuando el paciente en remisión en la visita basal la mantenga durante todas las visitas de seguimiento. Para estimar la proporción de pacientes que mantienen la remisión a los dos años con una exactitud de al menos un 6% se necesitarán 180 pacientes.
Discusión y conclusionesLa principal fortaleza del diseño es el empleo de la evaluación ecográfica como patrón oro para valorar la actividad inflamatoria.
The treatment goal in rheumatoid arthritis (RA) is to achieve remission, which is defined as the absence of clinical and analytical evidence of disease activity and progression of structural joint damage.1 Composite indices, such as the Disease Activity Score (DAS), its variant for 28 joints (DAS28) and the Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI), have been the most widely used in clinical practice to measure this condition. However, there are differences in validity between the indices, as patients are considered in remission more frequently using DAS28 than using SDAI, and patients who meet the criteria for DAS28 may have significant residual activity.2,3 In addition, it has been observed that a value considered as remission by the SDAI < 3.3 had a sensitivity of 57% and a specificity of 74% in predicting the absence of a Power Doppler (PD) signal in the joint ultrasound assessment as a definition of remission.4 This means that patients in clinical remission show a low level of inflammation that is not always easily detectable by clinical examination or does not show up in laboratory results. On the other hand, some patients not considered in remission according to the indexes show absence of joint inflammation on ultrasound, which can lead to unnecessary intensification or change in treatment.
In view of the heterogeneity in measuring remission, the ACR/EULAR criteria were published in 2011, defining remission by means of 2 options. A Boolean criterion with 4 variables (painful, swollen joints, CRP and global assessment of the patient) or by means of the SDAI. However, these criteria, which are used for clinical trials, are not widely used in clinical practice.5
The patient's global assessment (PGA) is the only measure that comes directly from the patient that is included in the different indices. In clinical practice, patients often provide a higher PGA score than would be expected, based on the clinical activity of the observed disease. This would indicate that PGA is not exclusively linked to disease activity, as is the case with the swollen joint count (SJC); the painful joint count (PJC) and acute phase reactants.6 It has been observed that many patients fail to meet the criteria for remission just because their PGA score is higher than 1 (on a scale of 0–10) and it has recently been proposed that increasing the PGA cut-off point to 2 would increase the number of patients classified as in remission, as well as the agreement with other variables. such as the physician's global assessment, without altering its effect on radiological progression or disability.7 Despite this change, some authors argue that the PGA should be excluded from the definition of remission, as it would provide results more related to key outcomes, such as radiological progression or functional impairment.8,9
However, the incorporation of the patient's perspective in the definition of remission is relevant, as this is increasingly important in decision-making, not only treatment decisions, and has a wide range of available measurement tools.10–12
Taking into account the existing literature and the consensus of a multidisciplinary group of professionals, involved in the care of patients with RA, the SUMAR project has sought a definition of remission that incorporates the patient's perspective within the instruments that can measure the activity of the disease. This has been achieved through the collaboration of different health care professionals to reach the definition, hence the name SUMAR. The definition of remission provided by SUMAR includes 3 variables: remission according to one of the composite indices of inflammatory activity (DAS-28 < 2.6 or SDAI ≤ 3.3); global assessment of pain; and HAQ-DI.13 However, this new definition of remission for RA should be assessed in clinical practice to verify its usefulness and relevance. The hypothesis is that patients who comply with the SUMAR definition of remission will have achieved more authentic remission, since it specifically includes patient-derived variables such as pain and disability, and this will translate into a greater persistence of remission than patients who do not meet this.
Finally, studies examining sustained remission are scarce, many of them with maintained remission limited to one year or less, and show highly variable maintenance percentages.14–18 A study with a 2-year follow-up is justified in order to estimate the proportion of patients who have continued in remission throughout this period and also to identify predictors of this maintenance.
The aim of this manuscript was to detail the objectives and methodology of the RA-Remission study, on the impact of the inclusion of global assessment of pain and functional capacity in the definition of remission and its maintenance in RA.
Objectives of the RA Remission studyPrimary objective- 1
To estimate during a 2-year follow-up the proportion of patients in sustained remission, according to the SUMAR definition, in a group of patients in remission, as well as according to the SUMAR definition, at baseline.
- 1
To estimate the proportion of patients in remission detected by PD at baseline and at 2 years’ follow-up in a group of patients in remission as defined by SUMAR at baseline.
- 2
To estimate the proportion of patients in sustained remission during a 2-year follow-up, considering different definitions of remission.
- 3
To compare the proportion of patients in PD remission at baseline and at 2 years’ follow-up between a group of patients in SUMAR remission and a group of patients in non-SUMAR remission at baseline.
- 4
To compare the proportion of patients who maintained PD remission over 2 years, between a group of patients in SUMAR remission and a group in non-SUMAR remission, but remission according to DAS28-PCR, at baseline.
- 5
To compare the proportion of patients who maintained PD remission over 2 years between a group of patients in SUMAR remission and a group of patients in non-SUMAR remission, but remission according to SDAI, at baseline.
- 6
To estimate the proportion of patients in sustained SUMAR remission, taking DAS28-PCR < 2.6 in the definition of remission, over 2 years of follow-up.
- 7
To estimate the proportion of patients in sustained SUMAR remission using SDAI ≤ 3.3 in the definition of remission, over 2 years of follow-up.
- 8
To identify predictors of sustained SUMAR remission during a 2-year follow-up.
- 9
To compare the rate of disease flare-ups during the 2-year follow-up between patients in SUMAR remission and patients in remission based on other criteria at baseline.
- 10
To compare the Rheumatoid Arthritis Impact of Disease (RAID) questionnaire score at baseline, at 12 months, and at 2 years of follow-up between patients in SUMAR remission and patients in remission according to other criteria at baseline.
- 11
To compare the Productivity and Activity Impairment Questionnaire (WPAI) score at baseline, at 12 months, and at 2 years of follow-up between patients in SUMAR remission and patients in remission according to other criteria at baseline.
- 12
To compare the use of healthcare resources, over a 2-year follow-up, between patients in SUMAR remission and patients in remission according to other criteria at baseline.
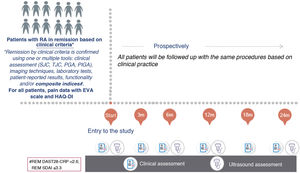
Longitudinal, prospective, observational, multicentre study (Fig. 1). The selection of participating centres was made through a public call among the members of the Spanish Society of Rheumatology (SER, in its Spanish acronym) and was based on the ability to include patients. Public hospitals were selected, with at least one nurse assigned to rheumatology and one rheumatologist with experience in the ultrasound assessment of patients with RA. The existence of a monographic consultation on RA/biologics was also assessed.
Study population. Selection criteriaInclusion criteria- -
Patients aged ≥18 years with RA, who meet the 2010 ACR/EULAR or ACR1987 classification criteria and have achieved clinical remission within the last 6 months, according to the clinical judgment of the treating rheumatologist. That is, patients have to be in clinical remission at the time of recruitment, and the duration of remission must be ≤6 months.
- -
Patients in functional class II or higher.19
- -
Patients with functional disability or chronic pain due to causes other than RA.
- -
Difficulty understanding the study questionnaires.
- -
Patients who are participating in a different research project, if this might hinder adequate participation in this study.
- -
In the opinion of the investigator, any other health problem that might hinder adequate patient participation in this study, as well as prospective follow-up.
Patients who meet the selection criteria will be invited to participate in the study during a routine medical appointment.
The study will be run in accordance with the principles of the Declaration of Helsinki in its latest revision, and has been approved by the Research Ethics Committee (REC) of the Hospital Universitario La Paz (the principal investigator's centre), as well as the REC of each participating centre that has requested it.
Patients will be asked to sign informed consent, which will be an essential requirement for their participation in the study.
Study visits and variables (Table 1)Follow-up will last 24 months and will consist of a baseline visit and follow-up visits at 3, 6, 12, 18 and 24 months (Fig. 1):
- -
Sociodemographic and lifestyle variables: age, sex, level of education and tobacco consumption.
- -
Anthropometric variables: height and weight.
- -
Comorbidities and extra-articular manifestations of RA.
- -
Date of diagnosis of RA.
- -
Date of commencement of referral.
- -
Existence of referrals prior to the current referral (Yes/No).
- -
Rheumatoid factor and anti-CCP antibodies.
- -
Structural damage to the joints due to RA (existence of narrowing of the joint space and/or marginal bone erosion in at least one metacarpophalangeal, proximal interphalangeal, carpal or metatarsophalangeal joint in recent hand and foot x-ray).
- -
Number of painful joints. Number of swollen joints.
- -
Laboratory test: GSV, CRP.
- -
Overall assessment of the disease by the patient on the day of the visit (visual numerical scale, from 0 = very good to 100 = very bad).
- -
Overall assessment of disease activity by the physician (visual numerical scale, from 0 = very good to 10 = very poor).
- -
Disease activity indices: DAS28, SDAI, CDAI.
- -
Global assessment of pain on the day of the visit (visual numerical scale, from 0 = no pain to 100 = the worst pain imaginable).
- -
HAQ-DI.20
- -
Impact of RA: RAID questionnaire.21
- -
WPAI questionnaire: to assess the deterioration in work productivity and daily activities.22
- -
Joint ultrasound assessment. This will be performed in each participating centre by a rheumatologist expert in ultrasound, blinded to the rest of the findings of the study. The PD signal will be set for 30 joints (carpal, metacarpophalangeal (1st to 5th MCP), proximal interphalangeal (1st to 5th. PIP) and metatarsophalangeal [2nd to MT5th]) and 6 tendon regions (carpal extensor tendons [2nd, 4th, and 6th extensor compartment]), using a semi-quantitative scoring method consisting of a scale from 0 to 3.23
Variables and study visits.
| Variables | V1 / baseline | V2 / at 3 months | V3 / at 6 months | V4 / at 12 months | V5 / at 18 months | V6 / at 24 months |
|---|---|---|---|---|---|---|
| Sociodemographic and lifestyle | X | |||||
| Anthropometric | X | |||||
| Comorbidities | X | |||||
| RA Diagnosis Date | X | |||||
| Referral Start Date | X | |||||
| Rheumatoid factor and ACPA | X | |||||
| Structural joint damage from RA | X | |||||
| N° painful joints. N° swollen joints. | X | X | X | X | X | X |
| Overall assessment of the patient's disease | X | X | X | X | X | X |
| Lab tests | X | X | X | X | X | X |
| Disease activity rates | X | X | X | X | X | X |
| N° of outbreaks since last visit | X | X | X | X | X | |
| Global assessment of pain | X | X | X | X | X | X |
| HAQ-DI Questionnaire | X | X | X | X | X | X |
| Overall assessment of disease activity by physician | X | X | X | X | X | X |
| Joint ultrasound assessment | X | X | X | X | ||
| Treatment | X | X | X | X | X | X |
| Changes in treatment between visits | X | X | X | X | X | |
| RAID Questionnaire | X | X | X | |||
| WPAI Questionnaire | X | X | X | |||
| Unscheduled visits to Rheumatology between visits | X | X | X | X | X | |
| Emergency admissions between visits | X | X | X | X | X | |
| Surgical interventions between visits | X | X | X | X | X |
- -
0 indicates absence of a PD signal.
- -
1 indicates up to 3 isolated, 1 confluent, and 2 isolated or 2 confluent PD signals.
- -
indicates PD signal > grade 1, but occupying less than 50% of the intraarticular area.
- -
indicates PD signal in more than 50% of the intraarticular area.
- -
0 indicates absence of PD signal.
- -
1 indicates peritendinous focal PD signal within the synovial sheath (i.e., signals in a single sheath area), observed in 2 perpendicular planes, excluding normal feeding vessels.
- -
indicates peritendinous multifocal PD signal within the sheath, observed on 2 perpendicular planes, excluding normal feeding vessels.
- -
Indicates diffuse peritendinous PD signal within the widened synovial sheath (i.e. signals filling the widened sheath), seen on 2 perpendicular planes, excluding normal feeding vessels.
- -
Presence of erosions in the ultrasound assessment of the baseline visit. Erosion is considered to be the existence of a solution of continuity in the cortical bone, visible on 2 perpendicular planes.24
- -
Treatment at the time of the baseline visit. If this is a biological agent, the line of treatment during which remission has been achieved.
- -
Changes in treatment between visits.
- -
Number of outbreaks since the previous visit. An outbreak will be considered in the case of DAS28-PCR > 3.2 and an increase of >0.6 in DAS28-PCR.25
- -
Outpatient visits in rheumatology not scheduled since the previous visit.
- -
Admissions to the emergency room from the previous visit.
- -
Surgical interventions from the previous visit.
- -
Nonsurgical hospital admissions for RA-related causes since the previous visit.
The following definitions of referral shall be used:
- -
According to SUMAR:
- •
In patients without structural damage: DAS28-PCR < 2.6 or SDAI ≤ 3.3, pain (numerical scale; 0–10) ≤2 and HAQ-DI ≤ 0.5.
- •
In patients with structural damage: DAS28-PCR < 2.6 or SDAI ≤ 3.3, increased pain from previous visit ≤1 and increase in HAQ-DI from previous visit ≤0.25.
- -
According to PD: sum of the PD signal of all joints and tendons explored ≤1.23
- -
According to activity indices: DAS28-PCR < 2.6 or SDAI ≤ 3.3.
For each of the definitions, sustained remission shall be considered when the patient in remission, according to that definition at baseline, maintains this remission during all follow-up visits.
Quality controlThe SER Research Unit will carry out online monitoring of all patients listed in the database, which will make it possible to check that there are no inconsistencies or missing relevant values. In addition, the monitor of the SER Research Unit will visit all the participating centres to carry out on-site monitoring of 50% of patients included in each centre, randomly selected.
Statistical analysisSample size: Based on the primary endpoint and the variable proportion of sustained remission found in the literature,14–18 to estimate the proportion of patients who maintain remission at 2 years with an accuracy of at least 6% will require 180 patients, providing the following accuracy for a 95% confidence level:
- -
4.4% accuracy if the proportion of patients who maintain remission at 2 years is 10%.
- -
5.8% accuracy if the proportion of patients who maintain remission at 2 years is 20%.
Assuming 10% loss to follow-up, 200 patients should be included. A total of 10 centres will participate in this study.
Analysis of resultsThe proportions will be estimated with a 95% confidence interval.
The comparison between proportions will be made using the Chi-square test.
Multiple logistic regression will be used to analyse predictive variables at baseline (demographic, clinical, and treatment variables) of sustained SUMAR remission over a 2-year follow-up. The selection of variables will be based on the recommendations of Hosmer DW et al., starting with simple logistic regression and considering those with a p-value below 0.20, also including variables based on interpretation or clinical interest.26 The procedure used in multiple logistic regression would be similar to forward selection, since the variables are introduced one by one and the results are checked. The selection will also take into account the principle of parsimony which will provide a useful and simple explanatory model. The p-value threshold used in multiple logistic regression will be 0.05.
To assess the effect of SUMAR remission at baseline on outcome variables throughout multiple follow-up visits, logistic, linear, or Poisson multiple longitudinal regression will be used. Potential confounding variables (identified among the different demographic, clinical, and treatment variables at baseline) will be taken into consideration. The selection of these variables will be carried out in accordance with the recommendations of Hosmer DW et al., starting with simple regression and considering those variables with p values below 0.20, also including variables based on interpretation or clinical interests.26 The procedure used would be similar to forward selection, since the variables are introduced one by one and the results are checked. The selection will also take into account the principle of parsimony which will provide a useful and simple explanatory model. The p-value threshold used in multiple regression will be 0.05.
DiscussionThis manuscript details the objectives and methodology of the Remission-RA study, which aims to evaluate the definition of remission, in clinical practice, established by consensus by a multidisciplinary group on the SUMAR project (SUMAR contributions from different health care professionals involved in the care of RA patients). The main contribution of this new definition would be a greater contribution from the patient's perspective, by incorporating the global assessment of pain and HAQ.
To this end, a cohort of patients in short-term remission (≤6 months), according to the clinical judgment of their rheumatologist, will be included and followed over a period of 2 years. This follow-up period will enable us to evaluate sustained remission for a longer period than that considered in most studies.14–18
The main strength of the design of this study is the use of ultrasound assessment as the gold standard for assessing inflammatory activity, since imaging tests have been described as a more accurate method than the use of clinical assessment alone for this assessment.1 In each of the centres, the ultrasound assessments will be carried out by a single rheumatologist who is an expert in running the test, and who will remain blinded to the rest of the information collected in the study.
On the other hand, the monitoring plan, both online and on-site in all participating centres, will seek to guarantee the quality of the data collected.
As a limitation of the design, it should be noted that in relation to the cut-off points in the global assessment of pain and the HAQ-DI to be used in the definition of remission, the SUMAR project proposed to use different thresholds for patients with and without structural damage, since it is known that structural damage is a permanent change that could prevent the normalisation of parameters, such as pain and disability. However, the concrete values were not defined.
The cut-off points used in this study have been selected after a narrative review of the literature. It has been reported that the best cut-off point to define an "acceptable" pain level in RA is ≤2.27 For the HAQ-DI, a value ≤0.528,29 was considered adequate functionality. These cut-off values will be used in patients with no structural damage. The wide variety of patients with structural damage makes establishing a single cut-off point difficult, so in the SUMAR project the absence of progression was established as a threshold. Taking into account the concept of minimally clinically important deterioration, these thresholds will be progression of pain from the previous visit ≤1 and progression in HAQ-DI from the previous visit ≤0.25.27,30,31
FundingThe Remission-RA study was funded by AbbVie, which provided input to the study protocol. AbbVie will not play any role in collecting, analysing, and interpreting the data collected in the study. They have not been involved in the drafting of this article or in the decision to submit the article for publication.
Marta Domínguez-Álvaro, Daniel Seoane-Mato and Javier Bachiller-Corral declare that they have no conflict of interest.
Virginia Ruiz-Esquide has received support for conference attendance, speaking fees and consulting from Abbvie, Lilly, UCB and Galapagos. Alejandro Balsa has received fees from Abbvie, BMS, Lilly, Galapagos, Pfizer, UCB, Novartis, Janssen, Nordic, Sanofi and Sandoz. Mercedes Alperi has received fees from Abbvie, BMS, Galapagos, Gebro Pharma, Janssen, Lilly, Nordic, Pfizer, Sanofi and UCB. Federico Díaz-González has received fees for presentations and scientific consulting from Abbvie, AlphaSigma, MSD, BMS, Novartis, Lilli, Janssen, Celgene, Hospira and Biogen/Samsung Bioepic. He has also received fees from MSD, Abbvie, ROCHE, Pfizer and Novartis.
To Irene Monjo from the Rheumatology Dept. of La Paz University Hospital, for her advice on the definition of the procedure for the ultrasound examination.
Paloma Vela, Alejandra Bermúdez, Irene Calabuig Salas, Irene Notario Ferreira, Rocío Caño, Silvia Gómez (Hospital General Universitario Dr. Balmis); Veronica García Garcia, Jesús Loarce Martos, Carlos Guillén, Javier Domínguez, Marina Hernández (Hospital Universitario Ramón y Cajal); Ana Ortiz, Esther Vicente (Hospital Universitario de La Princesa); Marta Loredo (Hospital Universitario Central de Asturias); Irene Monjo Henry, Chamaida Plasencia, Marta Novella (Hospital Universitario La Paz); Andrés Ponce (Hospital Clínic de Barcelona); Ana Lois, Jesus Carlos Fernández (Hospital Universitario de A Coruña); Dolores Mendoza, M. Dolores Ruiz Montesino, Manuel Maqueda López, Carmen Lopez (Hospital Universitario Virgen Macarena); Eduard Graell, Menna Rusiñol, Anna Carreras, Clara Feliu (Parc Taulí Hospital Universitari) y Lourdes Mateo, Susana Holgado Pérez, Anika Nack, Rodrigo Duran (Hospital Universitario Germans Trias i Pujol).