The patient was a 30-year-old woman with a recent diagnosis of systemic lupus erythematosus (SLE), which had been determined by the following classification criteria: malar rash, polyarthritis, World Health Organization (WHO) class III glomerulonephritis, confirmed by biopsy, lymphopenia and serology characterized by antinuclear antibodies tested with HEp2 1:2560 with a speckled pattern, anti-double stranded (ds)DNA antibodies tested with Crithidia luciliae 1:1.280, anti-Smith antibodies (+) 77U/mL (normal: <15U), C3 37mg/dL (normal: 20–50mg) and absence of anti-phospholipid (aPL) antibodies. Her disease activity index was 23 points calculated by SLEDAI. During her evaluation, a surface antigen positive for hepatitis B virus (HBV) was observed, and she was begun on only an intermediate dose of prednisone (0.5mg/kg/day) with no immunosuppressive therapy. Subsequently, a polymerase chain reaction (PCR) test that was negative for HBV ruled out the infection, and the patient did not keep her next appointment.
Two weeks later, the patient was admitted to the emergency department with a 7-day history of abdominal pain in the epigastric region, nauseas and vomiting. Vital signs at admission: 100/70mmHg, respiratory rate 25bpm, heart rate 116bpm and body temperature 37.5°C. The physical examination included abdominal distension and pain in the upper quadrant, with meteorism, and no presence of peristalsis, or rebound. She continued with serious evidence of SLE activity, documented by a SLEDAI of 9 (there were no dermatological, joint, hematological, hematuria or pyuria manifestations). Her laboratory results were indicative of normochromic normocytic anemia, leukocytosis and thrombocytosis. Among her liver function tests, amylase and the determination of azo compounds were within normal range.
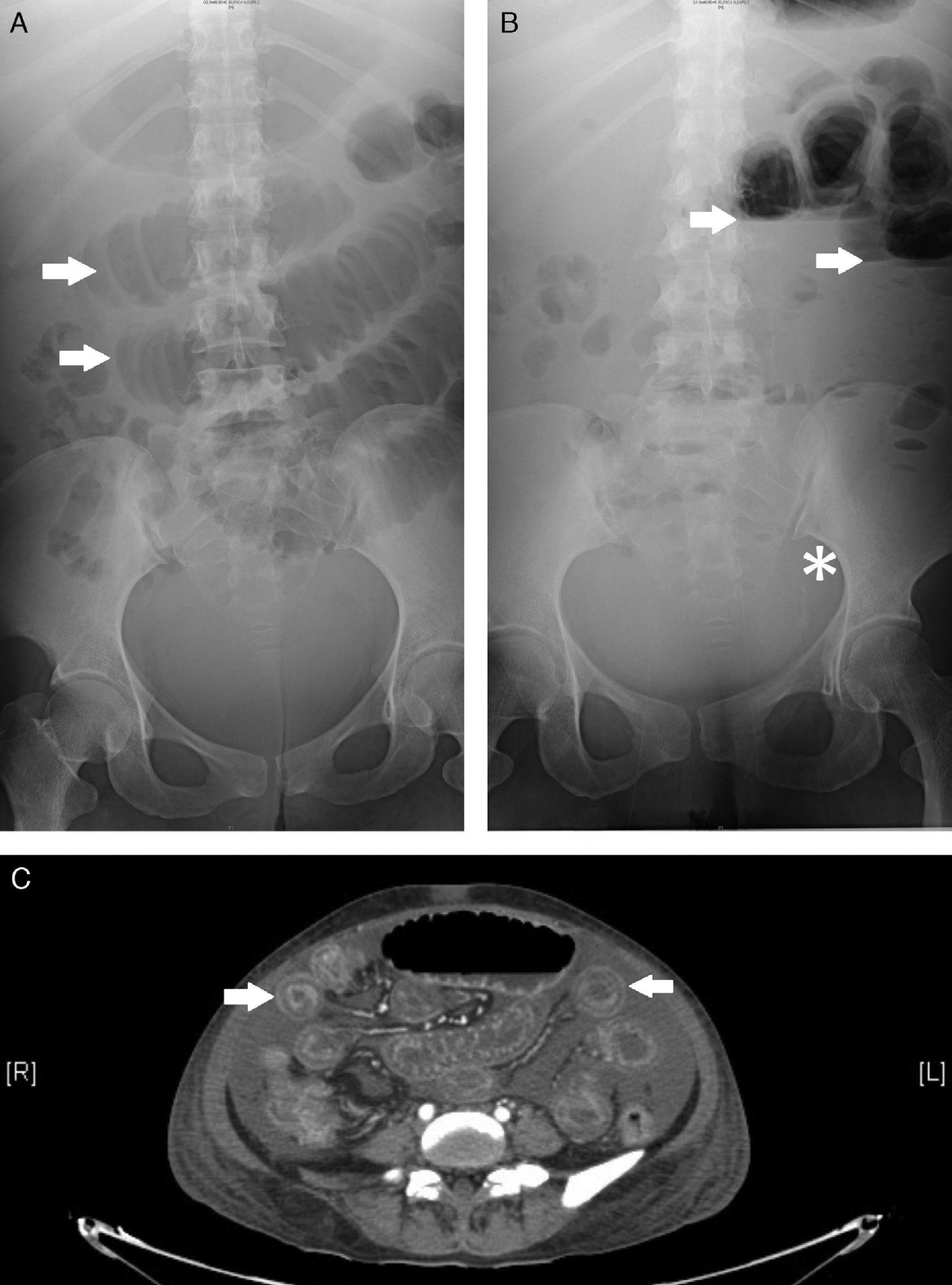
Plain abdominal radiographs were performed where there was evidence of dilated small intestinal loops, indicated by an image of a pile of coins (Panel A), as well as fluid and gas levels and the absence of distal gas (Panel B). Abdominal computed tomography diffusely confirmed small intestinal involvement, with multiple images of “target signs” (Panel C), which reflect swelling of the small intestinal wall, and an increased contrast uptake, with no evidence of intestinal perforation or obstruction, a sign that confirmed suspected lupus mesenteric vasculitis (LMV).
Treatment was begun with methylprednisolone (1g/3 days), with intravenous cyclophosphamide in accordance with Fauci1 (for a total dose of 600mg/3 days). If the symptomatology persisted, plasma exchange (PE) was indicated. After the first session, the patient began to improve, tolerating oral administration, with the development of peristalsis, as well as, a decrease in distension and the absence of abdominal pain. The patient underwent 4 more sessions, after which, she recovered totally from the clinical manifestations.
To the best of our knowledge, this is the first case of LMV, without aPL antibodies, treated with PE, which resulted in the complete improvement of our patient.
In agreement with the British Isles Lupus Assessment Group (BILAG, 2004), LMV is defined as vasculitis or inflammation of the small or large intestine, with histopathological or imaging findings that sustain the diagnosis. The overall incidence of LMV is 0.2%–9.7% among patients with SLE and it constitutes 29%–65% of acute abdominal pain in those individuals.2 The pathophysiological mechanisms proposed are vasculitis secondary to the deposit of immune complexes, whereas thrombosis of intestinal vessels is due to the presence of aPL antibodies.3 It mainly affects the territory of the superior mesenteric artery. The clinical findings, like those of the laboratory, are nonspecific and include abdominal pain, diarrhea and vomiting.4 Edema of the intestinal wall (91%), the “target sign” (71%), ingurgitation of the mesenteric vessels—the “comb” sign (71%)—and intestinal loop dilatation (24%),5 are tomographic findings described in LMV, but are not highly specific and can be observed in pancreatitis, mechanical intestinal obstruction, peritonitis and in inflammatory bowel disease.6 Other modes of evaluation include colonoscopy, ultrasound and scintigraphy. The treatment suggested for this serious manifestation of the disease consists of high-dose corticosteroids, an immunosuppressive agent and, in some severe cases, the patient should be considered for surgery. The prognosis of LMV depends on the extension of the vascular lesion, the early introduction of immunosuppressive therapy and, in some patients, the proper surgical evaluation.
We consider that, PE can complement the established treatment, in refractory cases. The safety and efficacy of this therapeutic modality should be determined in prospective, controlled clinical trials (Fig. 1).
Panel A demonstrates dilated small intestinal loops indicated by a pattern resembling a “pile of coins” (arrows). Panel B shows the fluid gas levels of the intestinal loops (arrows), as well as, the absence of distal gas (asterisk). Panel C shows multiple images of the “target sign” throughout the short intestine.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: González Padilla KA, Galarza Delgado DÁ, Garza Elizondo MA, Esquivel Valerio JA, Arana Guajardo AC. Imagen de «tiro al blanco» en una paciente con dolor abdominal y lupus eritematoso sistémico tratada con recambio plasmático. Reumatol Clin. 2016;12:348–350.