There are still controversies about the efficacy of cycling to a second tumor necrosis factor inhibitor (TNFi) in patients with inflammatory arthritis. The aim of this study was to evaluate survival, persistence and effectiveness of golimumab (GLM) in patients with rheumatoid arthritis (RA), axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) with previous experience with other TNFi and to compare these results with TNFi naive patients.
MethodsObservational cohort of consecutive patients with RA, PsA and axSpA who had started treatment with GLM according to medical indication. bDMARD naive and TNFi experienced patients were selected.
ResultsA total of 147 (62.3%) bDMARD naive and 45 (19.1%) TNFi experienced patients were included. Patients were followed up for a total of 441.5 patients/year, 55 (28.6%) discontinued GLM, 42 (28.6%) and 13 (28.9%) in each group, respectively (p=0.967). The majority (63.6%) suspended due to inefficacy, followed by lack of access (23.6%) and adverse events (9.1%). Median GLM survival was 74.0 months (95% CI 57.0, 91.0) and 71.0 months (95% CI 37.0, 105.0), in the bDMARD naive and TNFi experienced patients, respectively (p=0.695). Drug persistence at 6, 12, 24 and 36 months was 92.8%, 88.1%, 76.1%, 65.4% and 93.1%, 77.4%, 74.2%, 68.5%, respectively. In the multivariable analysis, having public health insurance was associated with higher risk of drug discontinuation (HR 2.56, 95% CI 1.28–5.00, p=0.008). TNFi experienced patients did not show significantly higher risk of GLM suspension (HR 1.35, 95% CI 0.70–2.57, p=0.370).
ConclusionIn this cohort, TNFi experienced patients had comparable survival and persistence of treatment with GLM. Having public health insurance was associated with lower drug retention rates.
Todavía existen controversias sobre la eficacia del cambio a un segundo inhibidor del factor de necrosis tumoral (iTNF) en pacientes con artritis inflamatoria. El objetivo de este estudio fue evaluar la supervivencia, la persistencia y la eficacia de golimumab (GLM) en pacientes con artritis reumatoidea (AR), espondiloartritis axial (EspAax) y artritis psoriásica (APs) con experiencia previa con otros iTNF y comparar estos resultados en pacientes sin tratamiento previo con iTNF.
MétodosCohorte observacional de pacientes consecutivos con AR, APs y EspAax que habían iniciado tratamiento con GLM según indicación médica. Se seleccionaron pacientes sin experiencia con DMARD biológicas y con experiencia en iTNF.
ResultadosSe incluyeron un total de 147 (62,3%) pacientes sin tratamiento previo con DMARDb y 45 (19,1%) pacientes con tratamiento previos con iTNF. Los pacientes fueron seguidos por un total de 441,5 pacientes/año, 55 (28,6%) descontinuaron GLM, 42 (28,6%) y 13 (28,9%) en cada grupo, respectivamente (p=0,967). La mayoría (63,6%) suspendió por ineficacia, seguida de falta de acceso (23,6%) y eventos adversos (9,1%). La supervivencia de GLM mediana fue de 74,0 meses (IC 95%: 57,0, 91,0) y 71,0 meses (IC 95%: 37,0, 105,0), en los pacientes sin tratamiento previo con DMARDb y en los pacientes con experiencia con iTNF, respectivamente (p=0,695). La persistencia del fármaco a los seis, 12, 24 y 36 meses fue del 92,8%, 88,1%, 76,1%, 65,4% y 93,1%, 77,4%, 74,2%, 68,5%, respectivamente. En el análisis multivariado, tener seguro de salud pública se asoció con mayor riesgo de discontinuación de GLM (HR 2,56, IC 95% 1,28-5,00, p=0,008). Los pacientes con experiencia con iTNF no mostraron un riesgo significativamente mayor de suspensión de GLM (HR 1,35, IC 95%: 0,70-2,57, p=0,370).
ConclusiónEn esta cohorte, los pacientes con experiencia previa con iTNF tuvieron una supervivencia y persistencia del tratamiento con GLM comparables. Tener cobertura de salud pública se asoció con tasas más bajas de retención de la droga.
Golimumab (GLM) is a human monoclonal antibody directed against TNFα in its soluble and transmembrane forms. It can be used subcutaneously or intravenously and has shown efficacy for use in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).1–3 Additionally, other four TNFα inhibitors (TNFi) are available for the management of these groups of diseases and are recommended by scientific societies for patients who fail to respond to conventional (c) treatments.4–8
However, some of the patients persist with high disease activity after the first TNFi or develop secondary failure with time. There is still controversy related to the use of a second TNFi in this population. Most current guidelines suggest using other biologic (b) including other TNFi, or targeted synthetic (ts) disease-modifying antirheumatic drugs (DMARDs) as appropriate.4–8 Recently, the American College of Rheumatology conditionally suggested in the 2021 RA management recommendation guide to switch the mechanism of action over cycling to the same drug class for patients receiving a bDMARD or tsDMARD who are not at target.4
The GO-AFTER study assessed the efficacy and safety of GLM in patients with active RA who had previously received one or more TNFi. After 6 months of treatment, an ACR 20, 50 and 70 response of 34%, 18% and 12%, respectively, was observed.9 Patients included in this type of study are strictly selected and do not reflect the characteristics of the entire population evaluated in daily practice, nor represent medication access problems or patient preferences.10 In this context, and taking into consideration the scarce evidence available regarding cycling mechanism of action after TNFi failure, the aim of this study was to evaluate survival, persistence and effectiveness of GLM in patients with RA, PsA and axSpA with previous experience with other TNFi and to compare these results with TNFi naive patients.
MethodsStudy design and patientsThis real-world registry (GO-REAL)11 is a multicenter, observational cohort including consecutive patients older than 18 years old with a diagnosis of RA (ACR/EULAR 201012 criteria), axial spondyloarthritis (ASAS 200913 criteria) or psoriatic arthritis (PsA) (CASPAR14 criteria), who started treatment with subcutaneous or intravenous GLM according to medical indication in five private and six public rheumatological centers from seven argentine provinces. Patients receiving GLM as first line bDMARD or second line after first TNFi suspension were selected for this analysis. On the contrary, those reporting previous treatment with more than one TNFi, other bDMARDs and small molecules were excluded. Data were obtained by review of medical records.
Patients were evaluated at baseline and followed up every six months from the start of the medication. Sociodemographic data, like age, sex, education, health insurance (social security, private and public health insurance) and clinical characteristics, including disease diagnosis and duration, musculoskeletal manifestations, comorbidities, smoking status and body mass index, were recorded. Additionally, previous treatment with non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, csDMARDs, bDMARDs and tsDMARDs, were registered. Regarding golimumab, the start date, route of administration, and concomitant medications were identified. Disease activity was assessed at baseline and every six months thereafter as follows: 28 or 66/68 tender and swollen joint count as appropriate, pain, patient and physician global assessment according to a numerical visual scale (mm) and erythrosedimentation rate and C-reactive protein. Later, composite indices were calculated, including DAS2815 for RA, DAPSA16 and MDA17 for PsA, and BASDAI18 for axSpA. Adverse events and their severity were recorded. They were classified as injection site reaction or during the infusion, gastrointestinal, hematological, or skin alterations, infection, and neoplasia. A serious adverse event was defined as those leading to death, threatening life, requiring hospitalization or prolongation of existing hospitalization, resulting in disability or permanent damage, or being associated with a congenital anomaly or malformation. In the case of treatment discontinuation, the date and reason were identified. Patients were followed until golimumab discontinuation, loss of follow-up, death, or study finalization in May 2022.
Ethical considerationsThis study was conducted in accordance with Good Clinical Practice (GCP) guidelines, the International Conference on Harmonization (ICH), and the ethical principles established in the Declaration of Helsinki. Personal identification data was coded and protected according to current national and international standards to guarantee confidentiality. The protocol was approved by an Independent Ethics Committee (CEI Claude Bernard, GOLIMUMAB.20230508.E). All patients who participated in this study signed the corresponding informed consent.
Statistical analysisDescriptive statistics were performed. Continuous variables were presented as mean and standard deviation (SD) for normal distributions, or median and interquartile range (IQR) (quartile 3 value–quartile 1 value) otherwise. Categorical variables are summarized as frequencies and percentages and were compared using the Chi-square test, and if assumptions were not fulfilled, categories were grouped using Fisher exact test. For continuous variables, Student's t test and Mann–Whitney U test were used as appropriate.
Kaplan–Meier curves were used to determine drug survival, as a surrogate of long-term effectiveness and safety. Comparisons based on the previous use of TNFi were made by log rank analysis. Variables associated with GLM survival (p<0.1) and those which were considered of interest according to published data were analyzed using Cox proportional regression model. Additionally, drug persistence was assessed at 6, 12, 24 and 36 months. A secondary analysis excluding those who suspended GLM because of lack of access to the drug was performed. Drug effectiveness was assessed as the percentage of patients achieving disease remission or low disease activity after 6 months. Remission was considered in RA as DAS28≤2.6, in PsA as DAPSA≤4 and in axSpA as BASDAI<4; while remission or low activity DAS28≤3.2, DAPSA≤14 and BASDAI<4, respectively. The incidence of adverse events (AE) was expressed as events per 100 patients/year.
A p<0.05 was considered significant. All statistical analyses were performed with R version 4.0.0 (Free Software Foundation, Inc., Boston, USA).
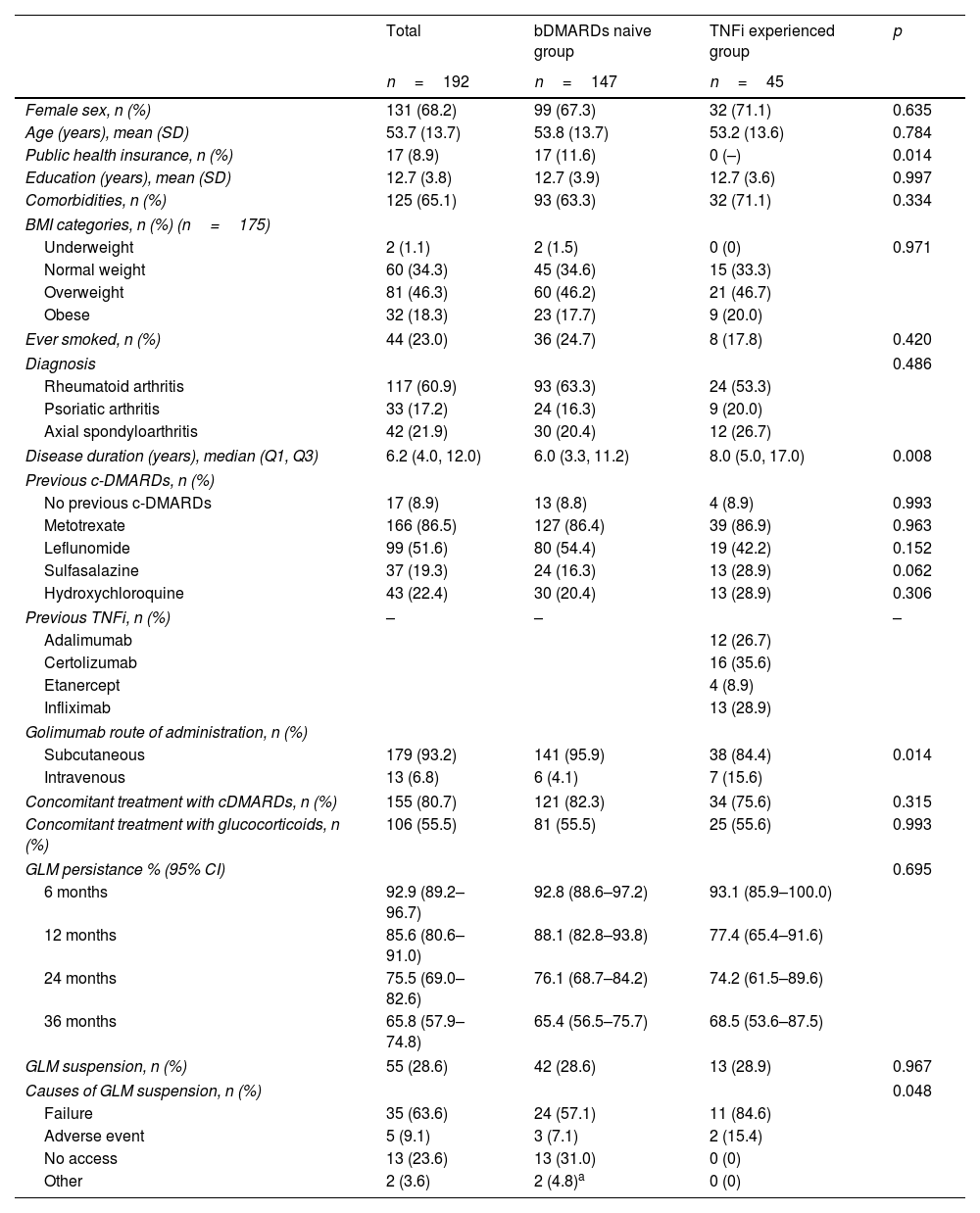
ResultsThe GO-REAL registry included 236 patients, 147 (62.3%) bDMARD naive patients and 45 (19.1%) TNFi experienced. The rest (44, 18.6%) were excluded from this analysis because they received GLM as 3rd or 4th ts/bDMARD line. RA was the most frequent rheumatic disease (60.9%), followed by axSpa (21.9%) and PsA (17.2%). Disease distribution was similar among groups, but disease duration was longer for those receiving GLM after TNFi suspension (median 8.0 years, IQR 17.0–5.0 vs median 6.0 years, IQR 11.2–3.3, p=0.008). All patients included in this group had social security or private health insurance (p=0.014) and presented comorbidities slightly more frequently (71.1% vs 63.3%, p=0.334). Regarding GLM, most of the patients used the subcutaneous route of administration (93.2%), but the TNFi experienced group received more frequently intravenous GLM (15.6% vs 4.1%, p=0.014). The use of concomitant glucocorticoids and cDMARDs was comparable between groups (Table 1).
Patient's demographic and clinical characteristics.
| Total | bDMARDs naive group | TNFi experienced group | p | |
|---|---|---|---|---|
| n=192 | n=147 | n=45 | ||
| Female sex, n (%) | 131 (68.2) | 99 (67.3) | 32 (71.1) | 0.635 |
| Age (years), mean (SD) | 53.7 (13.7) | 53.8 (13.7) | 53.2 (13.6) | 0.784 |
| Public health insurance, n (%) | 17 (8.9) | 17 (11.6) | 0 (–) | 0.014 |
| Education (years), mean (SD) | 12.7 (3.8) | 12.7 (3.9) | 12.7 (3.6) | 0.997 |
| Comorbidities, n (%) | 125 (65.1) | 93 (63.3) | 32 (71.1) | 0.334 |
| BMI categories, n (%) (n=175) | ||||
| Underweight | 2 (1.1) | 2 (1.5) | 0 (0) | 0.971 |
| Normal weight | 60 (34.3) | 45 (34.6) | 15 (33.3) | |
| Overweight | 81 (46.3) | 60 (46.2) | 21 (46.7) | |
| Obese | 32 (18.3) | 23 (17.7) | 9 (20.0) | |
| Ever smoked, n (%) | 44 (23.0) | 36 (24.7) | 8 (17.8) | 0.420 |
| Diagnosis | 0.486 | |||
| Rheumatoid arthritis | 117 (60.9) | 93 (63.3) | 24 (53.3) | |
| Psoriatic arthritis | 33 (17.2) | 24 (16.3) | 9 (20.0) | |
| Axial spondyloarthritis | 42 (21.9) | 30 (20.4) | 12 (26.7) | |
| Disease duration (years), median (Q1, Q3) | 6.2 (4.0, 12.0) | 6.0 (3.3, 11.2) | 8.0 (5.0, 17.0) | 0.008 |
| Previous c-DMARDs, n (%) | ||||
| No previous c-DMARDs | 17 (8.9) | 13 (8.8) | 4 (8.9) | 0.993 |
| Metotrexate | 166 (86.5) | 127 (86.4) | 39 (86.9) | 0.963 |
| Leflunomide | 99 (51.6) | 80 (54.4) | 19 (42.2) | 0.152 |
| Sulfasalazine | 37 (19.3) | 24 (16.3) | 13 (28.9) | 0.062 |
| Hydroxychloroquine | 43 (22.4) | 30 (20.4) | 13 (28.9) | 0.306 |
| Previous TNFi, n (%) | – | – | – | |
| Adalimumab | 12 (26.7) | |||
| Certolizumab | 16 (35.6) | |||
| Etanercept | 4 (8.9) | |||
| Infliximab | 13 (28.9) | |||
| Golimumab route of administration, n (%) | ||||
| Subcutaneous | 179 (93.2) | 141 (95.9) | 38 (84.4) | 0.014 |
| Intravenous | 13 (6.8) | 6 (4.1) | 7 (15.6) | |
| Concomitant treatment with cDMARDs, n (%) | 155 (80.7) | 121 (82.3) | 34 (75.6) | 0.315 |
| Concomitant treatment with glucocorticoids, n (%) | 106 (55.5) | 81 (55.5) | 25 (55.6) | 0.993 |
| GLM persistance % (95% CI) | 0.695 | |||
| 6 months | 92.9 (89.2–96.7) | 92.8 (88.6–97.2) | 93.1 (85.9–100.0) | |
| 12 months | 85.6 (80.6–91.0) | 88.1 (82.8–93.8) | 77.4 (65.4–91.6) | |
| 24 months | 75.5 (69.0–82.6) | 76.1 (68.7–84.2) | 74.2 (61.5–89.6) | |
| 36 months | 65.8 (57.9–74.8) | 65.4 (56.5–75.7) | 68.5 (53.6–87.5) | |
| GLM suspension, n (%) | 55 (28.6) | 42 (28.6) | 13 (28.9) | 0.967 |
| Causes of GLM suspension, n (%) | 0.048 | |||
| Failure | 35 (63.6) | 24 (57.1) | 11 (84.6) | |
| Adverse event | 5 (9.1) | 3 (7.1) | 2 (15.4) | |
| No access | 13 (23.6) | 13 (31.0) | 0 (0) | |
| Other | 2 (3.6) | 2 (4.8)a | 0 (0) | |
b: biologic; DMARDs: disease-modifying antirheumatic drugs; TNFi: tumor necrosis factor inhibitors; n: number; SD: standard deviation; BMI: body mass index; Q: quartile; c: conventional; GLM: golimumab.
Patients were followed up for a total of 442patients/year (median 20.0 months, IQR 37.0–8.3), 351patients/year for the bDMARD naive group (median 21.0 months, IQR 42.5–10.5) and 91patients/year (median 17.0 months, IQR 36.0–9.0) for the TNFi experienced group. During this period, 55 (28.6%) patients discontinued GLM, 42 (28.6%) and 13 (28.9%) in each group, respectively (p=0.967). The majority (63.6%) suspended due to inefficacy, followed by lack of access (23.6%) and adverse events (9.1%). While treatment failure was significantly more frequent among TNFi experienced patients, no access to the medication was more frequent in the bDMARD naive group, p=0.048 (Table 1).
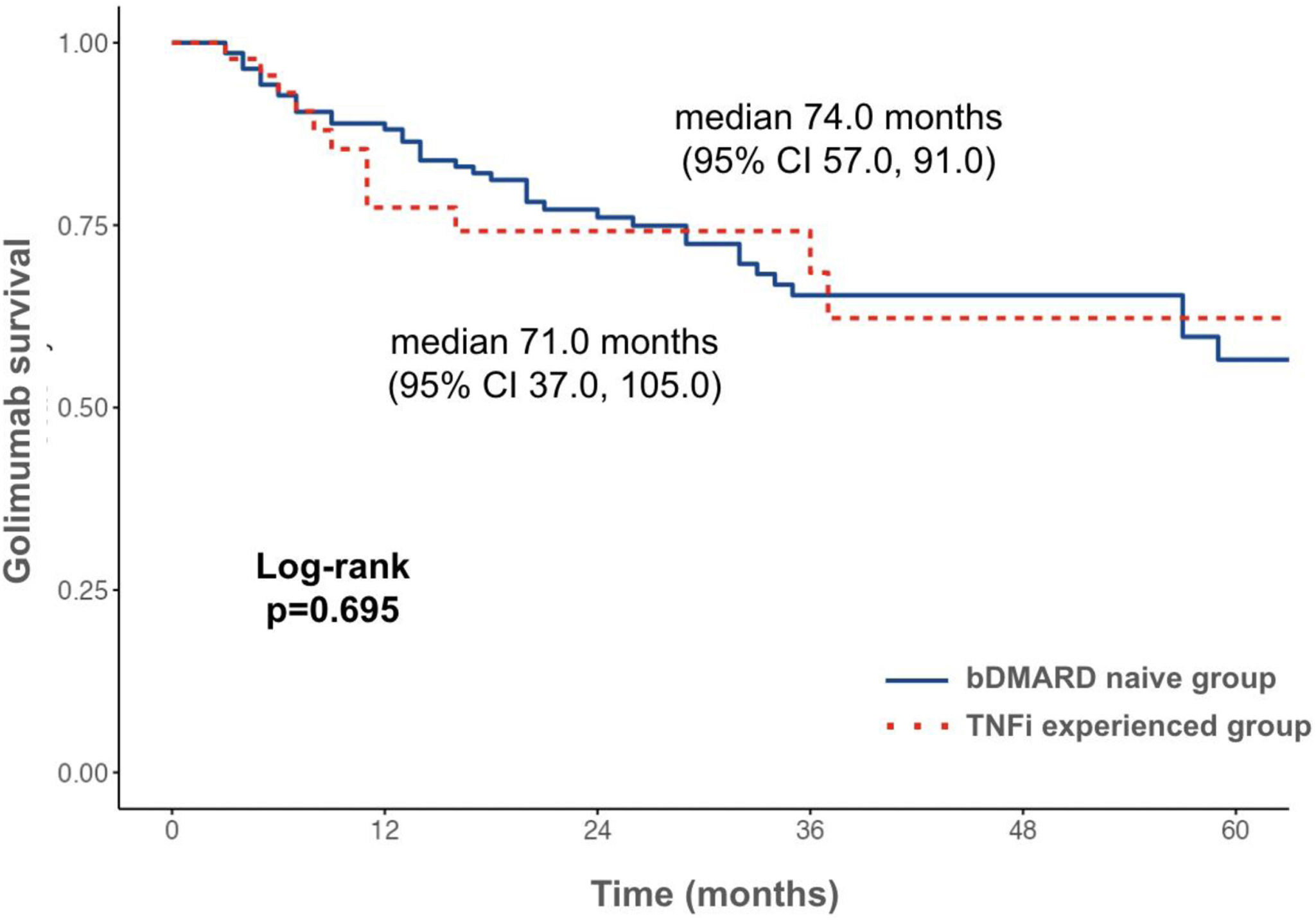
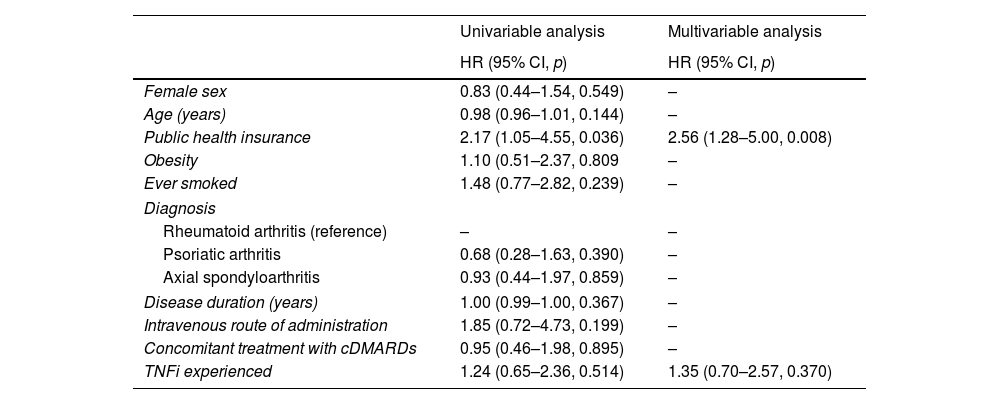
Overall drug survival was median 74.0 months (95% CI 59.0, 89.0) (Fig. 1) and GLM persistence was 92.9%, 85.6%, 75.5% and 65.8% at 6, 12, 24 and 36 months, respectively. When comparing groups, GLM survival was similar, median 74.0 months (95% CI 57.0, 91.0) and median 71.0 months (95% CI 37.0, 105.0), in the bDMARD naive and TNFi experienced patients, respectively (p=0.695) (Fig. 1). Likewise, drug persistence at 6, 12, 24 and 36 months was 92.8%, 88.1%, 76.1%, 65.4% and 93.1%, 77.4%, 74.2%, 68.5%, respectively (Table 1). In the sensitive analysis, excluding those who suspended GLM because of lack of access to the medication, the difference between groups in GLM survival increased, but remained not significantly associated (median 95.0 months, 95% CI 78.0, 112.0 vs 71.0 months, 95% CI 37.0, 105.0, p=0.240) (Supplementary Fig. 1). In the multivariable analysis, only having public health insurance was significantly associated with higher risk of drug discontinuation (HR 2.56, 95% CI 1.28, 5.00, p=0.008). TNFi experienced patients did not show significantly higher risk of GLM suspension (HR 1.35, 95% CI 0.70, 2.57, p=0.370) (Table 2).
GLM survival in bDMARD naive and TNFi experienced patients. Legend: Kaplan–Meier curve showing golimumab survival in patients bDMARD naive (blue) and TNFi experienced (red). bDMARD: biologic disease-modifying antirheumatic drugs; TNFi: tumor necrosis factor inhibitor; CI: confidence interval.
Factors associated with GLM suspension. Uni and multivariable analysis.
| Univariable analysis | Multivariable analysis | |
|---|---|---|
| HR (95% CI, p) | HR (95% CI, p) | |
| Female sex | 0.83 (0.44–1.54, 0.549) | – |
| Age (years) | 0.98 (0.96–1.01, 0.144) | – |
| Public health insurance | 2.17 (1.05–4.55, 0.036) | 2.56 (1.28–5.00, 0.008) |
| Obesity | 1.10 (0.51–2.37, 0.809 | – |
| Ever smoked | 1.48 (0.77–2.82, 0.239) | – |
| Diagnosis | ||
| Rheumatoid arthritis (reference) | – | – |
| Psoriatic arthritis | 0.68 (0.28–1.63, 0.390) | – |
| Axial spondyloarthritis | 0.93 (0.44–1.97, 0.859) | – |
| Disease duration (years) | 1.00 (0.99–1.00, 0.367) | – |
| Intravenous route of administration | 1.85 (0.72–4.73, 0.199) | – |
| Concomitant treatment with cDMARDs | 0.95 (0.46–1.98, 0.895) | – |
| TNFi experienced | 1.24 (0.65–2.36, 0.514) | 1.35 (0.70–2.57, 0.370) |
c: conventional; DMARDs: disease-modifying antirheumatic drugs; TNFi: tumor necrosis factor inhibitors.
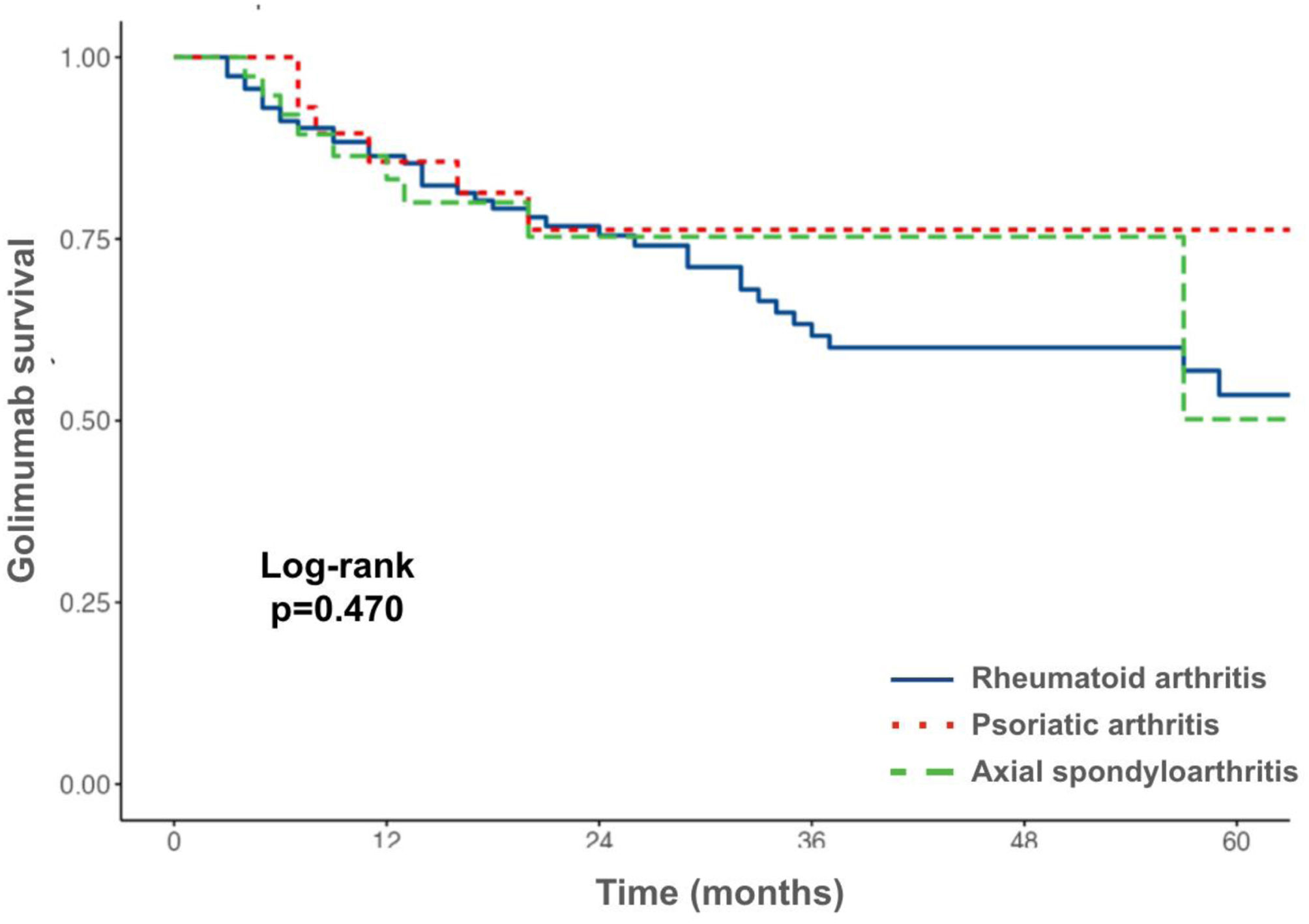
When comparing drug survival and persistence in different inflammatory arthritis groups, no statistical significant difference was observed (Fig. 2). Likewise, no difference between bDMARD naive and TNFi experienced patients was identified in RA patients, GLM survival and 6, 12, 24 and 36 months persistence was median 74.0 months (95% CI 35.0, 113.0) vs 71.0 months (95% CI 36.0, 106.0) and 91.2%, 87.7%, 74.7% and 60.6% vs 91.5%, 80.0%, 80.0% and 68.6%, respectively (p=0.940). Given the small number of patients, this analysis could not be performed in spondyloarthritis patients individually.
Regarding effectiveness, after 6 months of treatment with GLM, 27.9% of the patients from the bDMARD naive group and 22.2% of the TNFi experienced group were in remission (p=0.764). When remission or low disease activity was assessed, 35.4% and 37.8% achieved this outcome, respectively (p=0.969).
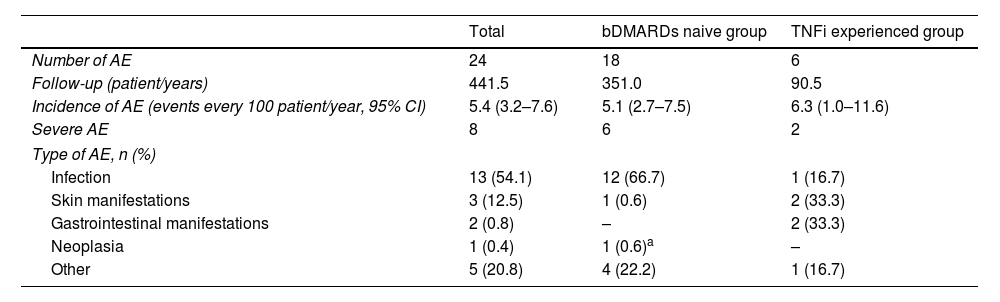
A total of 24 AE were reported in 21 (10.9%) patients during the follow-up period, which represents an incidence of 5.4 events every 100 patients/years (CI 95% 3.2–7.6). Eight (33.3%) of them were considered severe, 11 (45.8%) resulted in GLM suspension, but in only five (20.8%) of them was this definitive. The incidence of AE was comparable between groups, 5.1 (CI 95% 2.7–7.5) and 6.3 (CI 95% 1.0–11.6), in the bDMARD naive and TNFi experienced group, respectively (p=0.287). Types of AE are described in Table 3.
Safety of GLM.
| Total | bDMARDs naive group | TNFi experienced group | |
|---|---|---|---|
| Number of AE | 24 | 18 | 6 |
| Follow-up (patient/years) | 441.5 | 351.0 | 90.5 |
| Incidence of AE (events every 100 patient/year, 95% CI) | 5.4 (3.2–7.6) | 5.1 (2.7–7.5) | 6.3 (1.0–11.6) |
| Severe AE | 8 | 6 | 2 |
| Type of AE, n (%) | |||
| Infection | 13 (54.1) | 12 (66.7) | 1 (16.7) |
| Skin manifestations | 3 (12.5) | 1 (0.6) | 2 (33.3) |
| Gastrointestinal manifestations | 2 (0.8) | – | 2 (33.3) |
| Neoplasia | 1 (0.4) | 1 (0.6)a | – |
| Other | 5 (20.8) | 4 (22.2) | 1 (16.7) |
GLM: golimumab; b: biologic; DMARDs: disease-modifying antirheumatic drugs; TNFi: tumor necrosis factor inhibitors; AE: adverse event; CI: confidence interval.
This analysis of the GO-REAL registry of patients treated with GLM with long term follow-up showed good drug survival, persistence and effectiveness along all indications. This was true even in patients who had been treated with other TNFi.
Similar results were published in other real world studies. GLM persistence after one and two years varies between cohorts from 47% to 86% and from 40% to 77%, respectively.19–24 Particularly, the BIOBADASER registry showed a retention rate of 86% and 74% at 12 and 24 months, respectively. It was significantly greater when it was used as the first biological agent.25 Similar results were described by other cohorts, but they considered all biologics, not only TNFi.26,27 On the contrary, in the Norwegian registry NOR-DMARD and the Italian GISEA cohort, previous use of bDMARDs was not identified as a predictor of reduced GLM survival and persistence.28,29 In patients with RA who failed a previous TNFi in the LORHEN registry, a two year retention rate for GLM of 53.4% was observed.30 Results from a systematic literature review showed that GLM presented the highest retention rate as second line treatment compared to other TNFi.31This data is also supported by information provided by clinical trials. In long-term extension of the GLM study program, 5-year retention rate was around 70% when administered as first-line biological therapy in patients with RA, axSpA or PsA, and 40% when given after failure of other TNFi in RA patients.32–35 In our registry, GLM persistence at 12 and 24 months was 85.6% and 75.5% and although TNFi experienced patients showed slightly lower percentages, the difference was not significant.
Likewise, mean GLM survival was over 5 years and comparable between groups. As expected, TNFi patients had a longer disease at the time of GLM initiation. However, other variables that could affect TNFi survival, like type of rheumatic disease, smoking status, obesity and concomitant medication were balanced.36,37 On the contrary, TNFi experienced patients received GLM intravenously more frequently in our cohort. However this factor did not show a significant effect on GLM survival in the univariable, nor in the multivariable analysis.
As shown in other studies, the principal causes of drug discontinuation were treatment failure and development of AE.25 However it should be highlighted that one quarter of the patients who discontinued GLM in our cohort did so because of loss of access to the medication, showing that the health coverage was ineffective. In the same way, patients with public health insurance had 2.5 times higher risk of GLM discontinuation. This problem has already been observed in our country. Data provided by one public hospital showed that the lack of drug provision was responsible for bDMARD discontinuation in 28% to 41% of patients with RA, axSpA and PsA.38–40 While in a private center the prevalence was 15%.41 bDMARD naive patients presented higher frequency of public health coverage and events of drug discontinuation due to lack of access, which could affect the survival of GLM independently of drug effectiveness and safety. Consequently, a sensitivity analysis was performed and those who suspended GLM due to this reason were excluded. In the same way, no significant differences were identified in drug survival and persistence between groups.
Outcomes like drug persistence and survival combine indirect assessment of long-term effectiveness, safety and access. However, when effectiveness at 6 months was studied, 22.2% of the TNFi experienced group were in remission. These results are slightly lower than those described in the GO-BEYOND program, which pooled data from six observational studies in Europe. Remission at 6 months was between 45.5% and 58.3%, regarding the inflammatory arthritis.42 On the contrary, the GO-AFTER study showed that CRP-DAS28<2.6 at 24 weeks was achieved by 10% of the GLM treatment group.9
Our study has some limitations. Due to the observational design of this study, treatment selection bias could be present, since the indication of GLM or other drugs was made by each treating physician, considering access, characteristics and preferences of each patient. Data was obtained from medical records; for this reason, data collection bias could have affected our results. However, no variable presented over 5% of missing data, and imputation was carried out. Additionally, no active control group was included and comparisons between GLM and other bDMARD survival in TNFi experienced patients could not be performed. Although these results support the idea that TNFi cycling is an option, particularly when GLM is used, it could not be assessed if the mechanism of action switching is superior or not. On the other hand, there was no information available about the cause of discontinuation of the first TNFi, and thereby the effect of this variable on the second TNFi survival and effectiveness could not be established. In the same way, equal opportunity in getting a bDMARD treatment after physicians indication, accuracy of drug interval and missed doses were not assessed.
Of our knowledge, GO-REAL is the biggest real world registry of GLM treated patients in our country, and in Latin America and this is the first study which assesses GLM performance in TNFi experienced patients and compares these results with a bDMARD naive group. It was prospectively designed and a variety of efficacy and safety variables were recorded. Data from over 190 patients have allowed us to establish effectiveness, safety and survival of GLM in patients with inflammatory arthropathies. These data support the use of GLM in TNFi experienced patients and highlight a recurrent problem in our country, like in other parts of the world, where drug provision is not guaranteed.
- •
Data from this real world registry of patients with immune mediated arthritis treated with GLM showed good survival and was well tolerated, even in patients who had failed other previous TNFi.
- •
These results support the idea that TNFi cycling is an option, particularly when GLM is used.
- •
Lack of access to medication is an important cause of drug discontinuation in our country. Having public health insurance was associated with 2.5 fold risk in GLM suspension.
This study was conducted in accordance with Good Clinical Practice (GCP) guidelines, the International Conference on Harmonization (ICH), and with the ethical principles established in the Declaration of Helsinki, law 3301/09, and local guidelines. Personal identification data was kept anonymous according to national and international normatives. An independent ethics committee approved the protocol and informed consent form (CEI Claude Bernard, GOLIMUMAB.20230508.E).
FundingThis registry was developed and supported by the Argentine Rheumatology Foundation.
Authors’ contributionsAll authors listed in this manuscript were involved in drafting or revising this article critically for important intellectual content, and all authors approved the final version to be published.
Consent to participateAll patients signed the corresponding informed consent form to participate in this registry.
Consent for publicationIndividuals provided signed consent for the publication of their data.
Conflicts of interestCarolina A. Isnardi declares having received support for clinical investigation from Pfizer and for attending meetings from Montpellier and Abbvie. Agustín García Ciccarelli has received payment or honoraria for lectures, presentations, speakers bureaus or educational events from Janssen, Novartis and Abbvie. Ingrid Strusberg has received grants for her institution from Novartis, Abbvie, GlaxoSmithKline and Lilly, honoraria for lectures, presentations, speakers bureaus or educational events from Gema Biotech SAU and Janssen and support for attending meetings from Sandoz. Marcos Baravalle has received grants for her institution from Novartis, Abbvie, GlaxoSmithKline and Lilly, honoraria for lectures, presentations, speakers bureaus or educational events from Abbvie and support for attending meetings from Boehringer Ingelheim, Janssen, Novartis and Pfizer. Matias Palombo has received grants for her institution from Novartis, Abbvie, GlaxoSmithKline and Lilly. Carla Gobbi has received honoraria for lectures, presentations, speakers bureaus or educational events from Abbvie and Janssen, and support for attending meetings from Astra, Jansen and Novartis. Rodrigo Garcia Salinas has received consulting fees from Abbvie and Janssen, and honoraria for lectures, presentations, speakers bureaus or educational events from Abbvie, Novartis, Janssen, Pfizer, Lilly and Sandoz. Sebastian Magri has received honoraria for lectures, presentations, speakers bureaus or educational events from GlaxoSmithKline, Montpellier, Abbvie, Sandoz and Pfizer, and for participating in advisory boards form GlaxoSmithKline, Sandoz and Pfizer, and support for attending meetings from GlaxoSmithKline, Montpellier, Abbvie, Sandoz and Pfizer. Edson Velozo has received grants from Abbvie and GlaxoSmithKline, honoraria for speakers bureaus from Janssen, Novartis and Abbvie, for participating in advisory boards from Janssen, Novartis and Abbvie and support for attending meetings from Abbvie and Janssen. Enrique R. Soriano has received honoraria for lectures, presentations, speakers bureaus or educational events from Abbvie, Amgen, BMS, Janssen, Lilly, Novartis, Pfizer, Roche, Sandoz and UCB, for participating in advisory boards from Abbvie, Amgen, BMS, Janssen, Lilly, Novartis, Pfizer, Roche, Sandoz and UCB, and support for attending meetings form Abbvie, Astrazeneca, Pfizer, UCB and BMS. Gustavo Citera has received grants for his institution from Pfizer, consulting fees form Abbvie, Amgen, BMS, Boehringer Ingelheim, Janssen and Pfizer, honoraria for lectures, presentations, speakers bureaus or educational events from Abbvie, Amgen, BMS, Boehringer Ingelheim, Janssen, Pfizer, Raffo and Sandoz, or participating in advisory boards from Abbvie, BMS, Janssen, Pfizer and support for attending meetings form Abbvie and Janssen. The other authors have disclosed no conflicts of interest.