There is a lack of outcome measures for the assessment of physical activity in patients with axial spondyloarthritis (axSpA). For this matter, the modified Short QUestionnaire to Assess Health (mSQUASH) was developed and validated, originally in Dutch.
ObjectiveTo translate and cross-culturally adapt the mSQUASH into Spanish and to evaluate the equivalence of the translated version in patients with axSpA.
MethodsThe mSQUASH was translated following forward-backward procedure according to the protocol of Beaton. Two bi-lingual translators produced independent forward translations of the mSQUASH into Spanish, and the versions were harmonized in a consensual version. Another translator back translated the synthesized version into Dutch. A scientific committee reached consensus on discrepancies and developed a pre-final version of the questionnaire. The field test with cognitive debriefing involved 10 patients with axSpA with different gender, age, disease duration, educational level and working status.
ResultsThe translation process of the mSQUASH was completed without major issues. The first translation needed several iterations due to small discrepancies in the wording. Back-translation was performed without difficulties, and the scientific committee agreed upon a final version of the questionnaire. Cognitive debriefing showed the Spanish questionnaire to be clear, relevant, understandable and comprehensive. The preliminary version was accepted with minor modifications.
ConclusionsThe resulting Spanish version of the mSQUASH showed good linguistic and face validity according to the field test, revealing potential for use in clinical practice and research. In order to conclude the cross-cultural adaptation of the mSQUASH into Spanish, the next step is the assessment of psychometric properties of the Spanish version.
Las medidas de resultado para la evaluación de la actividad física en pacientes con espondiloartritis axial (EspAax) son escasas. Por ello, se desarrolló y validó el modified Short QUestionnaire to Assess Health (mSQUASH), originalmente en holandés.
ObjetivoDesarrollar el proceso de traducción y adaptación transcultural del mSQUASH al español, y evaluar la equivalencia de la versión traducida en pacientes con EspAax.
MétodosEl mSQUASH se tradujo siguiendo el procedimiento adelante-atrás según el protocolo de Beaton. Dos traductores bilingües realizaron traducciones directas independientes del mSQUASH al español, y las versiones se armonizaron en una versión consensuada. Otro traductor volvió a traducir la versión sintetizada al holandés. Un comité científico llegó a un consenso sobre las discrepancias y elaboró una versión pre-final del cuestionario. En las entrevistas cognitivas participaron 10 pacientes con EspAax de diferente sexo, edad, duración de la enfermedad, nivel educativo y situación laboral.
ResultadosEl proceso de traducción del mSQUASH se completó sin problemas mayores. La primera traducción necesitó varias iteraciones debido a pequeñas discrepancias en la redacción. La traducción inversa se realizó sin dificultades y el comité científico acordó la versión final del cuestionario. La evaluación cognitiva demostró que el cuestionario en español era claro, pertinente, comprensible y completo. La versión preliminar fue aceptada con pequeñas modificaciones.
ConclusionesLa versión española resultante del mSQUASH mostró una buena validez lingüística y aparente, revelando potencial para su uso en práctica clínica e investigación. Para concluir la adaptación transcultural del mSQUASH al español, el siguiente paso es la evaluación de las propiedades psicométricas de la versión española.
Axial spondyloarthritis (axSpA) is a rheumatic disease characterized by inflammatory back pain, and axial structural changes that may lead to impaired spinal mobility and limitations in daily activities.1 This negative impact on physical functioning poses a significant socioeconomical burden, and it is linked to productivity losses.2,3
International recommendations for the management of axSpA from the Assessment in SpondyloArthritis International Society (ASAS) and the European Alliance of Associations for Rheumatology (EULAR) advise regular physical activity for all patients.4 Physical activity has been associated with lower disease activity, and there is mounting evidence that different types of exercise also have positive effects on physical function, pain and mobility in patients with axSpA.5 Despite these promising benefits of exercise, previous findings suggest that patients with axSpA exhibit lower physical activity levels than healthy controls.6 In this sense, it is critical to reinforce the importance of physical activity in patients with axSpA and develop specific recommendations that are adapted to both patients and healthcare professionals.7
Physical activity may be assessed with measurement tools.8 Particularly, patient-reported outcome measures (PROMs) are easy applicable in daily clinical practice and research. In addition, information about the specific type of activity can be included. To fully benefit from these advantages, a questionnaire should include all daily physical activity domains and measure the duration, frequency and intensity of these activities.9 Moreover, psychometric properties, such as feasibility, validity, reliability and sensitivity to change, help evaluating the performance of these instruments.10,11 While there are several instruments that aim to assess physical function in axSpA, such as the Bath Ankylosing Spondylitis Functional Index or the Health Assessment Questionnaire, instruments that assess self-reported regular physical activity are scarce. In this regard, the Short QUestionnaire to Assess Health enhancing physical activity (SQUASH) is a physical activity questionnaire that was developed and validated in 2003 in the general Dutch population.12 Although it showed better performance on measuring daily physical activity in axSpA patients than the International Physical Activity Questionnaire (IPAQ), modification of the SQUASH was recommended to improve validity in patients with axSpA.13 Considering this, the modified-SQUASH (mSQUASH) was developed in Dutch in 2021, resulting in a feasible, valid, reliable and responsive questionnaire for the assessment of physical activity.14 Hence, the mSQUASH aims to tackle the unmet need on the assessment of the type and extent of daily physical activity in patients with axSpA, both in clinical practice and research.
This study aims to translate and cross-culturally adapt the mSQUASH into Spanish and to evaluate the equivalence of the translated version in patients with axSpA. The validation of the Spanish version of mSQUASH will provide a tool that allows to assess physical activity in patients with axSpA, both in clinical practice and in research settings.
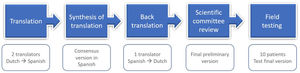
MethodsThe translation and cross-cultural adaptation study of the Spanish mSQUASH followed the current international recommendations, according to the Beaton method.14,15 This was done using the forward-backward procedure, which consists of five consecutive steps (Fig. 1). A scientific committee of the project was formed by an expert methodologist (VN-C), who provided guidance on the process of cross-cultural adaptation and ensured consistency of the methodology, one rheumatologist, and three Dutch-Spanish bilingual translators. The process of translation and cross-cultural adaptation was carried out between April and December 2021.
mSQUASH questionnaireThe mSQUASH contains five subdomains, namely commuting activities, activity at work or school, household activities, leisure-time activities, and sport-exercise. The domain of commuting activities has four items, the work or school, and household activities each have two items, the leisure-time domain has five items, and the sports-exercise domain can include up to four sports activities written in free text. The total score is calculated by taking a sum of the time spent active in each domain in total in minutes and multiplying by the intensity score (METs-value*demand factor). Higher scores indicate higher physical activity. The mSQUASH including codebook and syntax was published before.14
TranslationTwo bilingual translators (AJ, DPS, both native speakers for Spanish) produced independent forward translations of the mSQUASH, including item content, responses, and instructions, from Dutch into Spanish. One of the translators, who had a background in life sciences and rheumatic diseases, was informed of the conceptual content and the clinical background of the questionnaire (i.e., informed translator) whereas the other translator, who did not have a background in rheumatology, was not (i.e., uninformed translator). The different profiles of the translators contributed to ensure agreement and accuracy with the original Dutch version in terms of the terminology and the clinical content.16 An independent written report of the translations was written by each translator, highlighting any difficulties or uncertainties on the process.
Synthesis of translationAn online consensus meeting was held within the scientific committee including the two translators. The translations of the informed and uninformed translator were compared. After checking and discussing the discrepancies between translations, minor adjustments were made and both versions were harmonized in a consensual version. A written report documenting the synthesis of the two translations, specifying the concerns tackled and the final decisions.
Back translationAnother bilingual translator (GJ, native speaker for Dutch), who was blind to the original version of the questionnaires, back translated the synthesized version of the first Spanish translation of the mSQUASH into the original Dutch. He was not informed of the content or purpose of the questionnaire. Together with the Dutch back-translation, a translation report was made.
Scientific committee reviewThe scientific committee reviewed and assessed the back-translation and translation reports for conceptual, experiential, idiomatic and semantic equivalence in an online meeting. This ensured that items and words held the same meaning while capturing the experience of daily life in the Spanish culture. The scientific committee aimed to reach consensus on discrepancies across all versions and developed a pre-final Spanish version of the mSQUASH.
Patient interviews and cognitive debriefingThe pre-final version was assessed in a sample of 10 native Spanish patients with axSpA. A representative sample including patients from different socio-demographic backgrounds was included from the Rheumatology outpatient clinic of the La Paz University Hospital, Madrid. Patients were selected consecutively from the clinics, provided that they were representative of different ages and backgrounds. Inclusion criteria for these patients were (i) diagnosis of axSpA according to the rheumatologist and (ii) able to communicate verbally and in writing in Spanish. Patients were excluded in the presence of conditions that could potentially influence the assessment, such as illiteracy or dementia.
Patients filled mSQUASH and then had either a telephone or a face-to-face interview (cognitive debriefing) with one member of the scientific committee (DB). The cognitive debriefing interviews aimed to evaluate whether patients adequately understood the questionnaires. Besides, they were probed for their acceptability of the items in order to detect misleading items. Each respondent filled out the mSQUASH and was asked about each questionnaire item and response. The significance of the items as well as the responses were assessed, ensuring that the translated version was equivalent to the original questionnaire in a practical setting. Respondents were indicated that the purpose of the exercise was to test the questionnaire, not to assess themselves. Information on socio-demographic characteristics was collected.
ResultsThe Dutch mSQUASH served as the original version.14 The mSQUASH was translated and culturally adapted into Spanish. Appendix 1 includes the final version of the Spanish mSQUASH questionnaire.
Translation of the Dutch mSQUASH original versionBoth translators reported the initial independent forward translations as straightforward. Some discrepancies arose between them. In general, one of the translators used more colloquial terms; however, no major differences arose in most of the items when the two independent translations were compared. Items in which there were differences were discussed and agreed for a final version. An example of a minor discrepancy is reflected in the translation of the first domain, commuting activities. The original questionnaire reads “Andertransport”, which refers to the mean of transport and would be literally translated as “Otrostransportes”, as one of the translators reported. However, the other translator reported “Otrosdesplazamientos”. To overcome this difficulty, the context was discussed to translate into a semantically and conceptually equivalent domain. In fact, the conceptual meaning referred to the actual commute, and the domain was therefore left as “Otrosdesplazamientos”. Another example of a challenging translation was “Huishoudelijkeactiviteiten”; as reported by one of the translators, the actual translation would be “actividadesdomésticas”, but “tareasdomésticas” seems to better reflect the conceptual meaning and is more used in Spanish language in this regard, so it prevailed. Similarly, all discrepancies were agreed upon and a first version in Spanish was created.
Back translation of the preliminary version of the Spanish mSQUASHThe back translator reported no major doubts when interpreting the mSQUASH in Spanish. The back translation presented almost complete semantic, experiential and conceptual equivalence with the original version. There were minor discrepancies in the formulation of some items as compared with the original version that were discussed. As an example, item 8 was initially translated in the first version as “tareas domésticasextenuantes”. The word “extenuante” has some connotations in Spanish which surpasses the intensity of the actual meaning of the Dutch version and indicates heavily demanding work. For this reason, it was adapted as “tareas domésticas que requieren esfuerzointenso”, which better reflects the intensity referred in the original version. Another example that raised discussion, in the last domain (sports-exercise), was the word “bewegings-/oefentherapie”, which was initially translated as “terapia física/ocupacional”. However, it was agreed that in Spain an activity such as “bewegings-/oefentherapie” is not common practice, and a consensus for the equivalent “fisioterapia/rehabilitación” was reached, reflecting better the meaning of the original questionnaire.
Patient interviews and cognitive debriefingThe final Spanish version of the mSQUASH was tested in 10 patients with axSpA. Six patients had radiographic axSpA (r-axSpA) and four non-radiographic axSpA; 7 were male, 3 women; mean age (SD) was 38.9 (14.4) years. Table 1 summarizes the characteristics of the participants.
Individual patient characteristics of patients included in the cognitive debriefing.
| # | Gender | Age | Working status | Education | axSpA subtype | Disease duration | HLA-B27 | Drug | BASDAI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 63 | Working | University | r-axSpA | 35 y | + | NSAIDs | 2.3 |
| 2 | Male | 24 | Student | Secondary | r-axSpA | 6 y | + | NSAIDs | 0 |
| 3 | Male | 37 | Working | University | r-axSpA | 5 y | + | ADA | 2.5 |
| 4 | Male | 66 | Retired | University | r-axSpA | 23 y | + | IFN | 3.1 |
| 5 | Male | 29 | Working | University | r-axSpA | 11 y | + | ADA | 0 |
| 6 | Female | 26 | Working | University | nr-axSpA | 2 y | + | NSAIDs | ASDAS 0.9 |
| 7 | Male | 24 | Student | University | nr-axSpA | 1 y | + | ETA | 4.5 |
| 8 | Male | 35 | Working | University | r-axSpA | 12 y | + | GOL | 0 |
| 9 | Female | 40 | Working | Secondary | nr-axSpA | 4 y | + | NSAIDs | ASDAS 1.6 |
| 10 | Female | 45 | Unemployed | Primary | nr-axSpA | 9 y | − | GOL | 8.2 |
axSpA: axial spondyloarthritis; r-axSpA: radiographic axial spondyloarthritis; nr-axSpA: non-radiographic axial spondyloarthritis; y: years; NSAID: Non-steroidal anti-inflammatory drugs; ADA: adalimumab; IFN: infliximab; ETA: etanercept; GOL: golimumab.
All participants found the questionnaire clear and straightforward to complete and confirmed the adequate translation of most of the items. Minor spelling errors were corrected, and the response categories were homogenized following the comments of two of the respondents; in the physical demand categories, the explanation read “despacio/ligero”, whereas the responses read “lento/ligero”. Finally, the former prevailed. In addition, it was commented that the term “colegio” – translated literally from the Dutch “school” – was not comprehensive enough to reflect possibilities of places of education (i.e. it does not include university), so it was adapted to “el lugar de estudio”. Some other comments did not lead to any change as agreed by the scientific committee. Some patients indicated that options 14–17 would benefit of an indication that they meant to be self-completed, which is not present in the original questionnaire. Some participants commented that the bicycle was not used by them as a mean of transport; however, this is very dependent on the geography even within Spain. Moreover, some indicated that the sentence “no aplica” could be difficult to interpret, but this was attributed to the layout in a plain text document, not in the final format.
To sum up, in general the participants found the items of the Spanish version of the mSQUASH understandable and clear. The cognitive debriefing provided evidence for face validity.
DiscussionThis study provides a translated and culturally adapted Spanish version of the mSQUASH following the current international recommendations for this process.15,16 In the translation and back-translations, the meaning preservation and cultural adaptation were ensured. Only very minor modifications to the initial translation were implemented and no major cultural differences were highlighted. The back-translation discrepancies were minor despite the cultural and linguistic differences. The mSQUASH was completed by ten patients in the cognitive debriefing, which suggested good content validity without any patients finding the items unclear or irrelevant.
Patient-reported outcome measures assess disease outcomes from the patient perspective, including health-related quality of life, pain, activity limitations, satisfaction or adherence to treatment.17 They have been progressively more used in recent years,18 following their recognition by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a measure of treatment efficacy.19 In axSpA, PROMs have been frequently used to evaluate functional ability, quality of life, health status, or disease activity.19,20 However, one of the challenges in the development and selection of PROMs is the availability in the required language, which would ensure feasibility of using the instrument in that language.15 In this regard, cross-cultural validation refers to “the degree to which the performance of the items on a translated or culturally adapted instrument are an adequate reflection of the performance of the items of the original version of the instrument”.15 The first step towards a complete cross-cultural validation is to adapt the questionnaire to the translated language to provide conceptual, experiential, idiomatic and semantic equivalence. This was ensured in our study by a systematic five-step process of translation and back-translation, followed by a field testing of the pre-final translated version.21 This goes beyond a simple word by word translation of the questionnaire and aims to its adaptation to Spanish idiomatic expressions and culture. Of note, Spanish is the second most spoken maternal language in the world, after Mandarin Chinese, and it may be considered the third language for total number of speakers. While this translation was conducted in Spain, Spanish is also spoken in Hispanic America, Equatorial Guinea, Western Sahara and the Philippines, as well as by migrants in numerous countries such as the United States.
Physical activity is a construct of utmost importance in axSpA. Besides being a modifiable risk factor for cardiovascular disease, inactivity has been linked to higher disease activity and worse function.22 Thus, engaging in physical activity has been associated with better overall health and functioning.23 Considering this, a questionnaire to assess physical activity in axSpA is much needed to help promoting patient's tailored exercise. As the mSQUASH inquires for physical activity during an average week in the past month, this minimizes the weekly variation, as shown in the original validation by a high test–retest reliability.14 This is to add to the complete validation of the original mSQUASH, which showed to be a valid, reliable, sensitive to change and feasible questionnaire for the measurement of daily physical activity in axSpA patients.14
It is widely acknowledged that just translating a questionnaire into another language is insufficient for achieving cross-cultural validity, but there is no full agreement on the ideal strategy.24 In this regard, there are several guidelines for cross-cultural adaptation and methodology across them varies substantially.25 The majority of methods include committees, focus groups, and back translations. According to experts, most of these methods would attain similar results, and they could therefore be chosen following the preference of the authors. Currently available adaption methods use either a “forward–backward” translation or “forward only”. Beaton method, which employs “forward–backward” translation, was used in our study. In our experience this has proven to be beneficial, as backward translation raised further discussion on items, whose changes were later evidenced by the results. Besides, the field test with patients ensured that the translation was thorough and that the English and Spanish concepts were equivalent. Ten patients were involved in the field test. It may be argued that more patients would be beneficial, but given that this questionnaire has a limited number of items and aims to assess mainly one construct, it was considered that the sample of ten patients including a broad spectrum of socio-demographic background was convenient. This is in agreement with the field test of the health index for axSpA recently developed by ASAS.26
The present study is an important step for the world-wide use of the mSQUASH and its face validity. In this sense, a limitation of this study is that patients for cognitive debriefing were recruited in a single centre in Spain, which may hinder geographical variabilities in language and culture. However, the sample was representative of the axSpA population in terms of gender, age, educational level and working status. Besides, all participants involved in the translation, both from the scientific committee and patients, were from Spain, which may lead to some linguistic variations in some items in other Spanish-speaking regions of the world. This will need further research to confirm the validity of this Spanish version in some other Spanish-speaking countries. Of note, all patients in our study were diagnosed with axSpA, and therefore we cannot extrapolate our conclusions to other diseases. Besides, it would have been interesting to measure the time that patients needed to complete the questionnaire, to further assess the feasibility of the instrument. Of note, this study represents only the first step towards the complete validation of the instrument; before the Spanish mSQUASH can be implemented in clinical practice, it is critical to assess its psychometric properties in a larger group of patients.
ConclusionThe mSQUASH was successfully translated and adapted into Spanish, showing adequate face and content validity and ensuring the feasibility of its use. In this sense, the mSQUASH holds promise to be a useful tool to assess the amount and type of daily physical activity in axSpA for clinicians and researchers. Nevertheless, further steps towards the complete validation are needed before implementing the use of this instrument.
Authors’ contributionsDB: Study design and set up, Presentation of the data, Discussion and interpretation of the translations within the Scientific Committee, Cognitive debriefing with patients, Writing original draft. AJ, DPS: Translation into Spanish, Discussion and interpretation of the translations within the Scientific Committee, Manuscript revision. GJ: Back-translation into Dutch, Discussion and interpretation of the translations within the Scientific Committee, Manuscript revision. CP, SR, SA, AS, AB: Methodology, Discussion and interpretation of the findings, Manuscript revision. VNC: Study design and set up, Methodology, Discussion and interpretation of the translations within the Scientific Committee, Manuscript revision, Supervision.
Ethical approval and consent to participateThe Ethics Committee of Hospital La Paz was consulted and allowed the development study. The need for approval or informed consent was waived.
Consent for publicationConsent for publication from participants was obtained.
Availability of data and materialsThe data underlying this article will be shared on request to the corresponding author with permission of the scientific Committee of the project.
FundingNo funding was received for this work.
Conflict of interestsDiego Benavent has received speaker's honorarium from Jannsen, Roche, Grant/research support from: Novartis. Dora Pascual-Salcedo has received speaker's honorarium from Pfizer, Menarini, Takeda, Abvvie., Grant/research support from: Pfizer, Menarini, Takeda, Abvvie. Chamaida Plasencia-Rodríguez has received speaker's honorarium from Pfizer, Abbvie, Lilly, Sandoz, Sanofi, Biogen, Roche and Novartis, Grant/research support from: Pfizer and Abbvie. Sofia Ramiro has received speaker's honorarium from Eli Lilly, MSD, Novartis, UCB, Consultant of: AbbVie, Eli Lilly, MSD, Novartis, Pfizer, UCB, Sanofi, Grant/research support from: AbbVie, Galapagos, Novartis, Pfizer, UCB. Anneke Spoorenberg has been consultant of AbbVie, Novartis, Pfizer; UCB, Lilly, Grant/research support from: AbbVie, Pfizer. Alejandro Balsa has received speaker's honorarium from Pfizer, Abbvie, Lilly, Galapagos, BMS, Sandoz, Nordic Pharma, Gebro, Roche, Sanofi, UCB, Consultant of: Pfizer, Abbvie, Lilly, Galapagos, BMS, Nordic Pharma, Sanofi, UCB, Grant/research support from: Pfizer, Abbvie, BMS, Nordic Pharma, Gebro, Roche, UCB. Victoria Navarro-Compán has received speaker's honorarium from AbbVie, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB Pharma, Consultant of: AbbVie, Eli Lilly, MSD, Novartis, Pfizer, UCB Pharma, Grant/research support from: AbbVie and Novartis. Andrea Jochems, Gijs Jochems and Suzanne Arends declare that they have no conflict of interest.
We would like to thank the participants involved in the translation.