The current paradigm of the management of rheumatoid arthritis (RA) recommends achieving a state of remission or low disease activity through the treat-to-target strategy. Our study assesses adherence to this strategy.
MethodPatients with RA (ACR-EULAR 2010 criteria) were included. From each centre, 19 patients were randomly selected. Clinical histories (CH) were assessed by independent auditors, checking compliance with predefined quality criteria. The study was approved by ethics committees.
ResultsWe included 856 patients (mean age 54 years; 71% women). The use of a combined index (CI) was recorded in 61% of cases. Visits were recorded every 4 weeks using a CI in 4% of CH while attempts were made to achieve remission. Monitoring of disease activity every 6–8 months after reaching the target was recorded in 73% of cases.
ConclusionsThe implementation of the treat-to-target strategy is barely recorded in patients with RA in routine clinical practice.
El paradigma actual del manejo de la artritis reumatoide (AR) recomienda alcanzar un estado de remisión o baja actividad mediante la estrategia de «tratar por objetivos». Nuestro estudio evalúa la adhesión a esta estrategia.
MétodoSe incluyeron pacientes con AR (criterios ACR-EULAR 2010). De cada centro, se eligieron al azar 19 pacientes. Auditores independientes evaluaron las historias clínicas (HC), verificando el cumplimiento de criterios de calidad predefinidos. El estudio fue aprobado por los comités de ética.
ResultadosSe incluyeron 856 pacientes (edad media, 54 años; 71% mujeres). El uso de un índice combinado (IC) se recogió en el 61% de los casos. En el 4% de las HC se registraron visitas cada 4 semanas utilizando un IC mientras se intentaba alcanzar la remisión. La monitorización de actividad cada 6-8 meses tras alcanzar el objetivo se registró en el 73% de los casos.
ConclusionesLa implementación de la estrategia de «tratar por objetivos» apenas está registrada en pacientes con AR en práctica clínica habitual.
Rheumatoid arthritis (RA) is a systemic autoimmune disease whose main characteristic is a chronic inflammation of diarthrodial joints. In absence of a successful therapy, RA leads to irreversible destruction of articular structure, functional impairment and loss of quality of life.
With the advent of biologic therapy and the optimal use of conventional disease-modifying anti-rheumatic drugs (DMARDs), being methotrexate (MTX) the corner-stone of RA treatment, remission of disease or, at least, a state of low disease activity (LDA) are considered nowadays realistic goals in the management of RA, as different guidelines and recommendations suggest.1–3
Several studies have compared standard of care by personal rheumatologist's judgement deciding the changes in therapy versus thigh control and dynamic escalation of the therapy according to pre-specified therapeutic targets.4–7 These studies have demonstrated better outcomes with the so called “treat to target” (TTT) strategies. Thus, the current paradigm of optimal clinical care of patients with RA recommends reaching a state of remission or LDA of the disease, assessed by composite indexes of activity, by means of a tight control and a dynamic adjustment of available therapeutic options, using a TTT strategy.8,9
Although the TTT strategy is widely known and assumed by rheumatologists as beneficial and feasible,10–12 there are barriers in routine clinical practice that could hinder its wide implementation and the optimal management of RA patients. The objective of our study was to objectively assess the level of implementation of the TTT recommendations in routine clinical care of RA patients.
MethodsThis study is an audit of clinical records of patients diagnosed of RA and managed in Spanish rheumatology units.
All the rheumatology units of Spain were invited to participate in the study. Those units that accepted to participate sent to the research team a computerized anonymized list of adult patients fulfilling the 2010 ACR-EULAR criteria for RA13 and diagnosed of RA between January 1st, 2010 and December 31st, 2013.
In order to accomplish an adequate representation of the RA population, 19 patients were randomly chosen from the list provided by every department.
Independent auditors, specifically trained by the research team, assessed the clinical records, verifying the fulfilling of the TTT recommendations in the management of the patients. Clinical, epidemiological and demographic data were recorded from every patient.
The study was approved by the ethic committees of every participant hospital. Descriptive statistics was used for the presentation of the results.
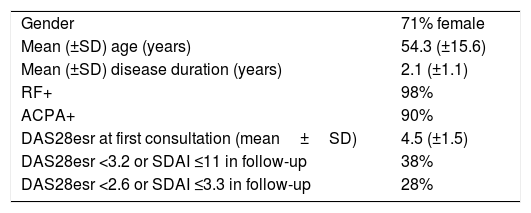
ResultsForty-six rheumatology units participated in the study. From them, a total of 856 clinical records, randomly chosen, were available for the audit. Table 1 shows the characteristics of the patients. Mean age of them was 54 years and 71% of patients were women. Mean duration of RA was 2 years.
Characteristics of the patients included in the study.
| Gender | 71% female |
| Mean (±SD) age (years) | 54.3 (±15.6) |
| Mean (±SD) disease duration (years) | 2.1 (±1.1) |
| RF+ | 98% |
| ACPA+ | 90% |
| DAS28esr at first consultation (mean±SD) | 4.5 (±1.5) |
| DAS28esr <3.2 or SDAI ≤11 in follow-up | 38% |
| DAS28esr <2.6 or SDAI ≤3.3 in follow-up | 28% |
Abbreviations: ACPA, anti-citrullinated peptides antibodies; DAS28esr, disease activity index based on erythrocyte sedimentation rate; RF, rheumatoid factor.
An explicit assessment of the disease activity as a determinant element considered to select the therapy was recorded in 32% of the CRs. Use of at least a composite disease activity score (DAS28, SDAI or CDAI) was recorded in 61% of the cases. In the follow-up, 38% of patients reached a state of low disease activity, defined by a DAS28 <3.2 and/or a SDAI ≤11.
In only 4% of the CRs, appointments every 4 weeks using a composite activity score had been recorded during the early stage while trying to reach remission after the diagnosis of RA.
Disease activity monitoring every 6–8 months after reaching the therapeutic target was recorded in 73% of the cases.
In 99% of the cases, the clinicians had registered patient's comorbidities and these associated conditions had been considered in planning the therapeutic approach and objective.
DiscussionOur data have shown a low implementation of the TTT strategy in the routine clinical practice of the rheumatology units participating in the study. The data are somewhat disappointing since the widespread dissemination of TTT strategy and the acceptance of their feasibility by rheumatologists14 would lead to the expectation of a wider implementation of this treatment strategy.
There are several barriers that have been suggested for the implementation of the TTT strategy.15 Likely, the main ones are the lack of time in real world clinical practice to use the compound indices of activity and the difficulty to get appointments for the patients with RA month by month during the escalation of medication to achieve a state of remission or, at least, LDA. A potential solution would be to maintain preferential citation slots in order to monitor patients every 1–3 months. This would require an additional effort in rheumatologist resources but, on the other hand, the TTT strategy has been shown to be more effective than usual care16 and even cost-efficient in the medium and long term.17
Although our results are quite disappointing, we used a very stringent criterion, four weeks, to judge whether the TTT strategy was being followed. Likely, the results would not have been so poor if we had used 3 months instead of 4 weeks as the criterion of excellence to adjust the treatment of patients with RA during the escalation of therapy in order to reach a state of remission or LDA.
In conclusion, a minority of the patients with RA are managed according to the TTT strategy, that is, using combined indices of activity to monitor the response to treatment and follow-up every 4 weeks until the therapeutic goal is achieved.
Ethical approvalThis article does not contain any studies with human participants or animals performed by any of the authors.
Compliance with ethical standardsThis study was funded by an unrestricted grant provided by the biopharmaceutical company AbbVie.
Conflict of interestsThe authors declare no conflict of interest. Ángel Gil de Miguel declares that he is Director of the Chair of Evaluation of Health Outcomes Universidad Rey Juan Carlos/Abbvie.
We thank to the Rheumatology units that accepted to be audited their help in the evaluation of clinical records and their commitment in the identification of areas of improvement in the management of RA patients.