Therapeutic advances in rheumatoid arthritis require periodic review of treatment guidelines.
ObjectiveTo update the Mexican College of Rheumatology guidelines on the pharmacological treatment of rheumatoid arthritis.
MethodBoard certified rheumatologists from different health institutions and regions of the country participated. Work teams were formed that reviewed the previous guidelines, elaborated new questions, reviewed the literature, and scored the evidence that was presented and discussed in plenary session. The conclusions were presented to infectologists, gynaecologists and patients. Recommendations were based on levels of evidence according to GRADE methodology.
ResultsUpdated recommendations on the use of available medications for rheumatoid arthritis treatment in Mexico up to 2017 are presented. The importance of adequate and sustained control of the disease is emphasised and relevant safety aspects are described. Bioethical conflicts are included, and government action is invited to strengthen correct treatment of the disease.
ConclusionsThe updated recommendations of the Mexican College of Rheumatology on the pharmacological treatment of rheumatoid arthritis incorporate the best available information to be used in the Mexican health care system.
Los avances terapéuticos en la artritis reumatoide obligan a revisión periódica de las guías de tratamiento.
ObjetivoActualizar las guías del Colegio Mexicano de Reumatología del tratamiento farmacológico de la artritis reumatoide.
MétodoParticiparon reumatólogos certificados de diferentes instituciones de salud y regiones del país. Se conformaron equipos de trabajo que revisaron las guías previas, elaboraron nuevas preguntas, revisaron la literatura y calificaron la evidencia, que fue presentada y discutida en sesión plenaria. Las conclusiones se comentaron con infectólogos, ginecobstetras y pacientes. Se emiten recomendaciones basadas en niveles de evidencia de acuerdo con la metodología GRADE.
ResultadosSe presentan recomendaciones actualizadas para el empleo de los medicamentos disponibles en México hasta 2017 para el tratamiento de la artritis reumatoide. Se enfatiza la importancia del control adecuado y sostenido de la enfermedad y se describen aspectos relevantes de seguridad. Se incluyen conflictos bioéticos y se invita a la acción gubernamental para fortalecer el tratamiento adecuado de la enfermedad.
ConclusionesLa actualización de las recomendaciones del Colegio Mexicano de Reumatología del tratamiento farmacológico de la artritis reumatoide integra la mejor información disponible para ser utilizada en el sistema de salud de México.
In August 2017, a committee of experts from the Mexican College of Rheumatology (CMR) met to update its guidelines for the pharmacological treatment of rheumatoid arthritis (RA). Rheumatologists and methodological advisors from various health institutions participated. Coordinators were assigned if the subject did not represent a conflict of interest. The analytical framework was defined with a review of the previous version,1 some questions were removed, others were kept or restructured, and others were added due to the availability of new treatments. The words MeSH (Medical Subject Heading) were identified; where these did not exist, the keywords for the search were included under the term “All fields”. The PICO methodology, which identifies population (P), intervention (I), comparator (C) and outcome (O), was used in the search. Randomised clinical trials, systematic reviews and meta-analyses over the period January 2012 to September 2017, published in English or Spanish, on an adult population were considered. In the event of lack of evidence, observational studies or other clinical practice guidelines were included as a source of information. We searched PubMed, the Cochrane Library and websites.
Articles were screened by title, abstract and finally by full text. Participants were trained in the GRADE methodology for standardising the quality rating of evidence and strength of recommendation.2 A face-to-face meeting was held in April 2018 to discuss the recommendations until reaching a consensus. The relevant sections were discussed with infectologists, obstetrician/gynaecologists and patients. The new version was posted electronically on the CMR website for 10 days for comments. The final version was integrated and the treatment algorithm was updated. The quality of evidence by GRADE was classified as very low, low, moderate, or high. Expert opinion was classified as very low quality.3 The strength of the recommendations was rated as strong or weak. Recommendations that were considered important but could not be rated in terms of quality of evidence or strength of recommendation were classified as “good clinical practice”.4
ResultsWhat are the general principles of the treatment of rheumatoid arthritis?- –
Diagnose in the initial stages.
- –
Treat appropriately.
- –
Management preferably by a rheumatologist.
- –
Aim to achieve and maintain remission or a low level of disease activity.
- –
Individualise treatment.
- –
Monitor periodically.
- –
Address comorbidities.
- –
Adjust management to clinical practice.
- –
Take decisions together with the patient.
The main objective of the treatment of RA is to achieve and maintain remission of the disease or at least a low level of disease activity taking the perspective of the patient into consideration.5–18
What is the efficacy of glucocorticoids in reducing pain and radiographic progression?Twenty articles were reviewed and seven were included: (1) glucocorticoids added to conventional synthetic disease modifying anti-rheumatic drug (csDMARD) therapy in early RA19; (2) comparison of triple therapy with csDMARDs vs methotrexate in monotherapy and with low doses of glucocorticoids20; (3) comparison of dexamethasone vs methylprednisolone in pulses for control of relapses21; (4) radiographic follow-up at 2 years of patients with early RA treated with decreasing glucocorticoids vs increasing doses followed by strict control22; (5) response to glucocorticoids at 2 weeks as a predictor of effectiveness of induction therapy with csDMARDs at 3 months23; (6) intra-articular glucocorticoids and csDMARDs in treat-to-target in early RA,24 and (7) usefulness of glucocorticoids to decrease radiographic progression in RA.25
Glucocorticoids can be used with different doses and administration routes in RA.22,23 Use of the lower doses for the shortest time possible is preferred.
Glucocorticoids in combination with csDMARDs can be used as “bridge” therapy to achieve rapid remission.19
In patients who do not achieve complete remission with csDMARDs, the use of intra-articular glucocorticoids may be considered, especially in cases of persistent monoarthritis.24
Potential long-term adverse reactions should be monitored.25
Recommendations- –
The use of prednisone≤10mg/day or equivalent is recommended as “bridge” therapy with csDMARDs in patients with a diagnosis of RA. High quality of evidence. Strong recommendation.
- –
In early RA (less than 6 months’ onset) with poor prognostic factors (seropositivity, high clinical activity, erosions) low doses of glucocorticoids (prednisone≤10mg/day or equivalent) are recommended to reduce disease activity and radiographic progression. High quality of evidence. Strong recommendation.
- –
The use of high doses (methylprednisolone pulses) of intravenous (IV) glucocorticoids is only recommended in patients with severe extra-articular manifestations (mononeuritis multiplex, rheumatoid vasculitis). High quality of evidence. Strong recommendation.
The evidence for this recommendation comes from studies of early RA whose aims were to achieve remission or low activity of RA and to arrest radiographic progression with the use of csDMARDs.
The results of one of the studies show that between 8.7% and 22.5% of the participants achieved remission and between 35.5 and 42.7% low disease activity at 3 and 6 months, respectively, with csDMARDs.26 Remission was observed at week 17 in 77% of the participants.27 Another study showed that the csDMARD group achieved remission in 49%, compared to 21% in the untreated group.28 Another publication29 reported that, in early RA, the use of methotrexate in combination with other csDMARDs was not superior to methotrexate in monotherapy in achieving remission, if glucocorticoids were used at the same time. Early remission predicts sustained clinical remission in patients with recent onset RA (OR=1.95; 95% CI: 1.02–3.74).30,31
Recommendation- –
We recommend the use of csDMARDs as soon as possible. High quality of evidence. Strong recommendation.
Methotrexate is still considered the cornerstone of treatment for RA. The recommendations are based on a meta-analysis of clinical trials that included 1432 leflunomide users, 922 methotrexate users, 133 sulfasalazine users, and 312 placebo users.32 The recommendation regarding the route of administration of methotrexate is based on a systematic review that included studies with different designs that compared the oral route with the parenteral route.33 The efficacy and toxicity of methotrexate are mainly related to the absorbed dose and not to the route of administration. Bioavailability is greater with parenteral methotrexate than with the oral route.33
Additional considerationsOral methotrexate is preferred to parenteral methotrexate due to its ease of administration
Recommendations- -
Methotrexate is recommended as the first line of treatment; when methotrexate is contraindicated we recommend leflunomide. High quality of evidence, strong recommendation.
- -
It is suggested that methotrexate should be started orally due to ease of administration. If doses>15mg/week are required, parenteral methotrexate should be considered. High quality of evidence. Strong recommendation.
A meta-analysis reported a higher probability of achieving ACR 50 with combined therapy (56%–67%) vs methotrexate as monotherapy (41%); additionally, patients who had not received methotrexate and who were treated with combination therapy had lower radiographic progression, and with triple therapy (methotrexate, sulfasalazine and hydroxychloroquine) a 61% probability of achieving an ACR score of 50 vs methotrexate and biologics, which had responses between 24% and 64%.34 There was a higher rate of adverse events (AE) in the combined therapy groups.34
Additional considerationsIn patients with RA who do not respond to csDMARDs in monotherapy, similar results are obtained with the use of triple csDMARD therapy (methotrexate, sulfasalazine and hydroxychloroquine) or combined therapy with methotrexate and biological DMARDs (BioDMARDs). In our setting, triple therapy should be the first option of combined therapy.35
Recommendation- -
We recommend the use of monotherapy with csDMARDs in patients with active RA with no previous exposure to DMARDS. Moderate quality of evidence. Strong recommendation.
- -
We suggest the use of combined therapy with csDMARDS in patients with active RA with poor prognostic factors or with previous failure with a csDMARD. Low quality of evidence. Weak recommendation.
The biological DMARDs (BioDMARDs) and JAK inhibitors available in Mexico are innovative anti-TNF drugs such as etanercept, infliximab, adalimumab, certolizumab pegol and golimumab; anti-IL6 (tocilizumab); abatacept, which is a costimulation blocker; and rituximab (anti-B cells). Biosimilars of etanercept and infliximab are also available. The JAK inhibitors are tofacitinib and baricitinib.
We considered the efficacy and safety data from clinical trials of BioDMARDs or JAK inhibitors in different clinical settings in RA. Early or established RA, without previous treatment or with inadequate response to csDMARDs or biological therapy, their use in monotherapy or in combination with csDMARDS were taken into account. Since the indications and their precautions are similar, they are described in a general manner and special considerations with some of them are mentioned separately. The assumption that the efficacy of different biological therapies and JAK inhibitors is similar comes not only from indirect comparisons of clinical trials conducted,36–39 but also from direct comparison studies: ADACTA, AMPLE, EXXELERATE, ACTION, ORAL STRATEGY, RA-BEAM.40–45
The use of BioDMARDS or JAK inhibitors in patients with established RA, with moderate to intense clinical activity, who have had insufficient response to csDMARDS has proved effective. Similar percentages are reported in ACR responses 20, 50 and 70, with average responses of 70%, 40% and 25%,36 respectively, at 24 weeks of treatment and reduction of disease activity by DAS28≥1.2 up to 70% at 2 years.38,39 The rates of ACR response 20, 50 and 70 are slightly lower: 60%, 30% and 15%, respectively, when the population has a history of a failure to respond to biological therapy. In patients with early RA, the clinical responses are better, with low disease activity achieved in up to 40% of patients and remission in 25%, maintained for up to 5 years.36 Similarly, controlled clinical and extension trials have shown that biological therapy or with JAK inhibitors, combined in both cases with csDMARDs (generally methotrexate), is superior to monotherapy for arresting structural damage.36–39 In general, the best clinical responses are achieved by administering these treatments in combination with methotrexate, especially in the case of anti-TNF.46–49
Some studies with tocilizumab and JAK inhibitors have shown that monotherapy treatment is superior to methotrexate and equivalent to therapy in combination with methotrexate.50,51
There is unequal availability in Mexico's health institutions of these therapeutic options, since availability is limited in the Ministry of Health and the Seguro Popular health initiative. It would be desirable for all institutions to have some of these drugs, with a different mechanism of action and administration route, for patients with moderate to high disease activity, without response to treatment with csDMARDs at appropriate doses over sufficient time.
Additional considerationsNo BioDMARD has an obvious therapeutic advantage over another.
Recommendations- -
We recommend the use of BioDMARDs or JAK inhibitors in patients with moderate to high RA activity with inadequate response to treatment with csDMARDs. High quality of evidence. Strong recommendation.
- -
In patients who have failed to respond to the first anti-TNF, a second anti-TNF can be used or they can be changed to a BioDMARD with a different mechanism of action. High quality of evidence. Strong recommendation.
- -
We recommend a change of mechanism of action in patients with RA with moderate or severe disease activity with inadequate response to BioDMARDS or JAK inhibitors. High quality of evidence. Strong recommendation.
- -
We recommend the use of tocilizumab or JAK inhibitors in patients with RA with moderate to severe disease activity in whom monotherapy is decided. High quality of evidence. Strong recommendation.
- -
In patients who require biological treatment, the decision about therapeutics with approved biological products (innovative or biocomparable) should be dictated by an individual risk/benefit assessment and not based solely on economic aspects. Good clinical practice.52
Infections. In patients with RA who receive ant-TNF, a higher risk of infections has been described, and some severe.53 The adjusted risk of any serious infections, compared with the use of csDMARDs, ranges from 1.1 to 1.8,4,55 which seems to be higher during the first year.56 British and Japanese registers show that etanercept could have a lower risk of serious infections than the monoclonal antibodies.38,57,58 The evidence indicates that patients that use anti-TNF should be closely monitored for the risk of infections, that they should be treated early when they acquire an infection, and that these drugs should be discontinued while the infection is active.
Tuberculosis (TB). The adjusted risk of TB in patients with RA using anti-TNF ranges from 2.7 to 12.5.54 All patients should be screened for TB, and those with a diagnosis of latent or active disease should receive the appropriate treatment. Retrospective studies have shown that it is safe to restart anti-TNF in patients receiving appropriate treatment for latent or active TB59,60 (Table 1).
Tuberculosis screening and management.
| Recommendation | Quality of evidence | Strength of recommendation |
|---|---|---|
| Tuberculosis screening (TB) | ||
| • TB screening is recommended by taking a clinical history asking about a history of living with people with active TB, physical examination, tuberculin test (or IGRA) and chest x-ray, in all patients with RA to be protocolised for the use of BioDMARDs or JAK inhibitors. | Very low | Good clinical practice |
| • It is suggested that screening should be repeated annually in patients who are under treatment with BioDMARDs and JAK inhibitors, given the risk of reactivation of latent TB or de novo infection. | Very low | Good clinical practice |
| • It is recommended that prophylactic TB therapy should be started in all patients presenting positive PPD≥5mm or positive IGRA, regardless of whether they have been BCG-vaccinated or not, and in COMBE positive patients with chest x-ray and signs suggestive of TB before starting therapy with BioDMARDs (especially with anti-TNF type drugs)61,62 or JAK inhibitors | Very low | Good clinical practice |
| Start of treatment with BioDMARDs or JAK inhibitors in patients who are receiving prophylaxis or treatment for established TB | ||
| • In patients with latent TB it is recommended to start or restart treatment with BioDMARDs or JAK inhibitors after one month of treatment with antituberculous drugs62,63 | Very low | Good clinical practice |
| • In patients with active TB it is recommended to start or restart BioDMARD/s or JAK inhibitors on completion of antituberculous treatment | Very low | Good clinical practice |
| • Patients with latent TB with positive tuberculin or IGRA results require clinical follow-up for appropriate diagnosis in case of reactivation, since repeated tests do not help in diagnosing recurrent TB | Very low | Good clinical practice |
Opportunistic germs. As for other specific infections, recent studies do not show an increased risk of infection by herpes zoster virus,54,64 and a systematic review found a greater risk of opportunistic infections.64
Reactions to application. These occur between 5.8% and 53.8%; most are mild or moderate and in very few cases require the drug to be discontinued.65
Cancer. Several recent meta-analyses have concluded that there is no increased risk of cancer associated with the use of anti-TNF drugs in patients with RA.54–68 A 2012 meta-analysis found a slight increase in non-melanoma skin cancer, which has not been confirmed in more recent meta-analyses and population studies.55,58,66,67,69 There is evidence for a slight increase in the risk of melanoma (aHR 1.5).54
The use of adalimumab or certolizumab during pregnancy appears to be safe; recent studies show that the use of these drugs is not associated with a higher risk of teratogenicity, miscarriage or foetal death compared to that expected for the general population.70–72
RituximabThe most relevant safety aspects of the use of rituximab are associated with its therapeutic effect and route of administration. Study of the long-term safety of rituximab with follow-up to more than 11 years summarises information from 8 controlled clinical trials, 2 long-term extension studies and one prospective observational study with experience in 14,816 patients/year. Most discontinuations of rituximab occurred in the first two infusion cycles; the main reason for discontinuation was not associated with the safety of the drug (22%) and only 7% discontinued due to adverse events (AE). Most of these AE occurred in the first 6 months and the frequency and nature of the events did not change throughout follow-up. There were 78 deaths (.58 patients/year) in the rituximab group, and 7 in the placebo group (.63 patient/year). The most common infections were respiratory and similar to the rest of the BioDMARDs.58 The frequency of infections did not increase with follow-up time or the number of applications. The most frequent severe infection was pneumonia (2%). The incidence of severe infections by opportunistic microorganisms was infrequent (.05 events/patients/year). Two cases of uncomplicated pulmonary TB were reported, and one new case of hepatitis B. Myocardial infarction had a rate of .39events/100 patients/year, which is similar to that described in patients with RA. No evidence of an increased risk of the development of neoplasms was found. The most frequent solid tumour was breast cancer, and its frequency does not represent an increase with respect to the benchmark population with RA.73
Due to the inhibition of B-lymphocytes (CD20) and the secondary decrease of immunoglobulins, measurement of IgG, IgM and IgA levels prior to the administration of each cycle of rituximab is recommended. European guidelines on the use of rituximab recommend monitoring immunoglobulin levels, with close monitoring for the possibility of infection in patients with low IgG levels.74
Rituximab increases the risk of reactivation of hepatitis B (OR: 7.2; 95%CI: 5.3–9.9), and therefore its administration in these patients should be monitored and its use avoided in cases of active infection.75
Progressive multifocal leukoencephalopathy has been associated with the administration of rituximab in 9 cases out of 351,396 RA patients treated with rituximab until 2015. The incidence rate of leukoencephalopathy in RA patients receiving rituximab is considered to be 2.56 per 100,000 patients.
Special consideration: rituximab can be used as a first choice of treatment in patients with a history of cancer.
AbataceptAlthough abatacept has been shown to have a better safety profile than other biologics in the treatment of RA, hepatitis B (HBV) deserves special mention. This has been analysed in several studies; in one study, 72 patients received abatacept; 47 were inactive carriers, 21 were occult, and 4 were chronic carriers of HBV, they all had normal baseline liver function tests or undetectable levels of HBV DNA, except those with chronic active hepatitis; 13 patients received prophylaxis with lamivudine and 4 with adefovir or tenofovir, and after 316 follow-up visit none of the patients had reactivation of hepatitis B, and there were no related AEs of interest.76
One study showed an association of older patients with a higher rate of discontinuation due to AE per 100 patients/year, especially severe infections (1.73 in the very young, 4.65 in the middle-aged, 5.90 in the elderly and 19.38 in the very elderly, P<.001).77 In 219 patients with abatacept combined with methotrexate in a long-term extension study, 114 (52.1%) completed 7 years, and safety was consistent with the initial study periods. Another long-term extension study conducted in patients with RA who were non-responders to methotrexate, received subcutaneous (SC) abatacept, identified 1385 patients who completed the double-blind stage; 1372 entered the long-term extension study and 945 (68.8%) completed ≥5 years of treatment. During the extension study, 97 (7.1%) patients discontinued treatment due to an AE. The incidence rate (event/100 patients/year of exposure) of AE of interest was: serious AE 7.73 (95% CI: 6.96–8.58), infection 38.60 (95% CI: 36.24–41.12), severe infections 1.68 (95% CI: 1.35–2.07), malignancy 1.09 (95%CI: .84–1.42), and autoimmune disease 1.33 (95%CI: 1.05–1.69), remaining stable over time. No association was observed between immunogenicity and worsened safety or loss of efficacy of abatacept. This study at 5 years established the safety of SC abatacept (125mg/week), showing consistent safety and lasting efficacy in the long-term treatment of patients with RA and inadequate response to csDMARDs.78 Although the presence of anti-drug-antibodies associated with abatacept has been demonstrated, these are not neutralisers and are not associated with a loss of efficacy, as seen with other biological drugs.79 Based on the above, we believe that abatacept maintains constant safety, as well as sustained efficacy over 7 years in patients with established RA and inadequate response to methotrexate,80 with fewer discontinuations due to AE (3.8% vs 9.5%), severe AE (1.6% vs 4.9%) and serious infections (0/12 vs 9/19 patients) than the comparison group.42
The use of abatacept has not been associated with interstitial lung disease.81
TocilizumabTocilizumab IV and SC as monotherapy or in combination with other csDMARDs has generally been well tolerated in clinical trials and in clinical practice after 9 years’ experience of the treatment.
The most common AE (>10%) were respiratory infections and hypercholesterolaemia.82 In clinical trials at 6 months the rate of infections with tocilizumab IV (8mg/kg) plus csDMARDs was 127events/100 patients/year, with a rate of severe infections of 5.3events/100 patients/year. In long-term exposure the general infection rate with tocilizumab was 108events/100 patients/year, and that of severe infections, 4.7events/100 patients/year.82
The most common AE were “infections and infestations” with a frequency of 7%–43% in the monotherapy group, and 6%–42% in the combined treatment group, nasopharyngitis being the most common. The drug was discontinued due to an AE in 6.2% of the patients, 9% in monotherapy, and 5.5% in combination with csDMARDs. The reasons for discontinuing treatment were skin and subcutaneous disorders with monotherapy (1.4%), and laboratory abnormalities in combination with csDMARDs (1.1%). The drug was discontinued due to lack of efficacy in 29 patients (1.6%). In addition, headache (7%) and arterial hypertension (6%) have been reported.82,83
Cases of diverticular perforation have been reported. Clinical trials at 6 months show rates of .26/100 people/year, and long-term exposure shows similar rates of .28/100 people/year.82
In studies with post hoc analysis and in long-term studies of treatment with tocilizumab IV (average of 3.7 years of follow-up) was associated with a cardiovascular event rate of 3.4events/100 people/year, with an average time for the first event of 680 days. A multivariate analysis found that the predictors of CVD were: advanced age (HR: 1.07), history of heart disease (HR: 2.32), higher DAS28 (HR: 1.36) and higher total cholesterol to HDL cholesterol ratio (HR: 1.33).82
Severe AE that occurred in between 3% and 5% of the total population studied were pneumonia, herpes zoster, increased aminotransferases, hypersensitivity and erythema at the puncture site.83 In addition, Dyslipidaemia/hypercholesterolaemia and gastrointestinal disorders hove been reported: pain, distension and intestinal haemorrhage, and folliculitis.84
Liver AE had a rate of .78events/100 patients/year. Of the patients, 2.5% had to discontinue the drug due to elevation of aminotransferases.85
Tocilizumab should not be used during pregnancy. There is a risk of miscarriage and foetal death with exposure to high doses in animals; the risk to humans is not known with certainty. Of the prospective and retrospective cases, 21.7% and 28.7% resulted in miscarriage, respectively. Malformations have been reported in 4.5% and premature births in 31.2%.82
JAK inhibitorsTofacitinib and baricitinib share safety aspects that are important to monitor.
Because these drugs are of recent use in our setting, many recommendations set out here are based on expert advice and not necessarily on information from pivotal studies. Clinicians should become familiar with the following general recommendations on JAK inhibitors:
Infections. These are the most frequently reported AEs. Most are usually mild or moderate, upper respiratory (14%–17%), urinary tract (3.4%–11%) and bronchitis (up to 11%) are the main infections.86–88 Herpes zoster has been reported in 3.9% of patients treated with baricitinib and in 4.39% of those treated with tofacitinib in the global programme, and 3.39% in Latin America.86,89 The frequency of severe infections ranges from 1.1% to 3.1%.40,89 Although opportunistic infections (specifically TB) have been reported in patients treated with JAK inhibitors, the frequency tends to be the same as for non-anti-TNF BioDMARDs. In general, the following risk factors have been described for infections: age>65 years, comorbidities such as diabetes mellitus, obesity and chronic obstructive pulmonary disease, and the use of prednisone>7.5mg/day.88,90 Discontinuing administration of these drugs is suggested if moderate or severe infection is suspected or confirmed, and it should be restarted once it has been resolved.
Lymphopenia. Lymphopenia≤500cells/ml3 can confer an increased risk of serious infections. It is suggested that treatment with JAK inhibitors should be temporarily or permanently discontinued if this figure is reached.40,90
Alterations in liver function tests. Elevations of ALT and/or AST levels have been observed with JAK inhibitors, but elevations ≥3 times (1.4%) occur infrequently and are generally asymptomatic and transient. They are more frequent in combination therapy with methotrexate. Periodic surveillance is recommended.86,88,91–93
Lipid profile and cardiovascular safety. Elevated total cholesterol, LDL cholesterol and HDL cholesterol has been described, with no changes in the total cholesterol/HDL ratio, in up to 49% of patients treated with JAK inhibitors, and is more frequent during the first 3 months of treatment.94 These alterations are usually reversed with statins.95 The clinical relevance of long-term cardiovascular risk is unknown.
- •
Thrombosis. There is an event of special interest in the safety profile of baricitinib, due to an increase in deep vein thrombosis and/or pulmonary thromboembolism. Special caution is advised with the use of baricitinib in patients with risk factors for deep vein thrombosis or pulmonary thromboembolism. If any of these events occur, baricitinib should be discontinued, promptly assessed and appropriate treatment started. The presentations of 2mg and 4mg every 24h have been approved in Mexico.96 However, the USA's Food and Drug Administration (FDA) have only approved the 2mg presentation, taking events of thrombosis into account.97
- •
Other laboratory alterations. These have been described with both drugs and very infrequently can cause anaemia, neutropenia, non-significant creatinine elevation86,92,93 and transient elevations of CPK. Up to 2% of patients who receive barcitinib present thrombocytosis.
- •
Neoplasias. To date, JAK inhibitors have not shown an increased risk of neoplasia. However, as with all other drugs, long-term follow-up is recommended with international registers and strict monitoring in the event of high suspicion.98,99
- -
We recommend strict monitoring of patients with RA receiving BioDMARDs or JAK inhibitors for the appropriate diagnosis and treatment of infections. High quality of evidence. Strong recommendation.
- -
The use of BioDMARDs or JAK inhibitors is not recommended in patients with active infection. High quality of evidence. Strong recommendation.
- -
We recommend considering other treatment options in patient with a high risk of recurring infections. High quality of evidence. Strong recommendation.
- -
We recommend monitoring AE related to the safety profile and mechanism of action of each BioDMARD or JAK inhibitor. High quality of evidence. Strong recommendation.
- -
We recommend screening and monitoring for skin cancer in patients with RA under treatment with BioDMARDs. High quality of evidence. Strong recommendation.
Consistently, the information shows a higher relapse rate in patients with RA in whom treatment with csDMARDs or BioDMARDs100–105 is reduced or discontinued. The incidence of relapse is lower with a decrease in dose than with discontinuing BioDMARDs. The incidence of relapse in patients following decrease of csDMARDs was 41%, and of BioDMARDs 37%.100
Risk factors for relapseThe following are considered predictors of relapse: positivity of anti-PCC or rheumatoid factor, longer duration of disease, and late start of treatment. Another predictor of relapse is the degree of remission, intense remission with DAS28<1.98 conferring a lower risk; serological markers have also been proposed, such as levels of interleukin-6 and matrix metalloproteinase-3, as well as the detection of synovitis with Power Doppler by musculoskeletal ultrasound.102,104,106–111
Recommendations- -
We suggest optimising treatment in patients in sustained clinical remission (at least 6 months). Optimisation should be individualised and consensual. The logistics of the hospital should be taken into consideration.1,6,106,112,113 Moderate quality of evidence. Weak recommendation.
- -
We suggest sequential reduction of the treatment under consideration to start drugs that generate a greater risk of AE in the short or long term, and an increase in costs.100,101,106,113 It is desirable to start glucocorticoid reduction and discontinuation. Moderate quality of evidence. Weak recommendation.
- -
We suggest reducing the dose from 20% to 50% of csDMARDs or BioDMARDs, or increasing the period of application, in patients who have remained in remission (DAS28<2.6 or equivalent) for at least 6 months.5,104,112–116 Moderate quality of evidence. Weak recommendation.
- -
We suggest discontinuing BioDMARDs in patients with early RA or established RA if the patient is in remission, preferably intensive (DAS28<1.98) for at least 6 months, if they are receiving the minimum dose of BioDMARDs and when there is no evidence of progression on imaging tests.114,116–119 Moderate quality of evidence. Weak recommendation.
- -
We recommend strict monitoring to detect relapse, at least every 12.16 weeks, and measuring disease activity by standardised scales.6,102,113,114 Moderate quality of evidence. Weak recommendation.
- -
In the event of a relapse, we recommend adjusting the dose or interval of the DMARDs with the aim of achieving remission again.113,114,116 Moderate quality of evidence. Weak recommendation.
The treatment of RA in pregnancy should be team managed where the patient, the obstetrician/gynaecologist and the rheumatologist maintain adequate communication before, during and after the pregnancy. Disease activity, therapeutic modifications, treatment options in the case of RA activity during gestation, puerperium and lactation should be carefully assessed in the preconception clinic. Disease control should be prioritised and the potential risks to the mother and foetus from both the use and discontinuation of a drug must be considered. Table 2 summarises the recommendations in pregnancy, lactation and fertility.
Pregnancy and lactation and fertility.
| Discontinuation of DMARDs in planned pregnancy | |||
|---|---|---|---|
| Drug and references | Time of discontinuation prior to conception | Quality of evidence | Strength of recommendation |
| Methotrexate129–133 | At least 3 months | High | Strong |
| Leflunomide131,132,134,135 | At least 2 years or cholestyramine wash-out | High | Strong |
| Rituximab136,137 | 12 months | High | Strong |
| Tocilizumab133,136,138,139 | 3 months (5 half lives) | Low | Weak |
| Abatacept132,140,141 | 10 weeks | Low | Weak |
| Anti-TNF132,142–146 | Can continue | Low | Weak |
| Tofacitinib132,136,145 | 2 months | Low | Weak |
| Control of clinical activity in pregnancy | ||||
|---|---|---|---|---|
| Drug and references | Recommended, suggested or contraindicated | Gestational stage | Quality of evidence | Strength of recommendation |
| Prednisone132–134,147–149 | Recommended | Entire pregnancy | High | Strong |
| Antimalarials150 | Recommended | Entire pregnancy | High | Strong |
| Sulfasalazine≤2g/day+5mg/d folic acid151 | Recommended | Entire pregnancy | High | Strong |
| Azathioprine132,134 | Recommended | Entire pregnancy | High | Strong |
| Certolizumab pegola,152 | Suggested | Entire pregnancy | High | Weak |
| Infliximab | Suggested | First 20 weeks | Low | Weak |
| Adalimumab72 | Suggested | Entire pregnancy | Moderate | Weak |
| Etanercept | Suggested | Up to week 30 | Low | Weak |
| Golimumab | Contraindicated | Entire gestation | Very low | Weak |
| Anti-TNF biocomparable | Contraindicated | Entire gestation | Very low | Weak |
| Treatment during lactation in patients with active RA | |||
|---|---|---|---|
| Drug and references | Additional considerations | Quality of evidence | Strength of recommendation |
| Prednisone130,149 | Dose 7.5–10mg | High | Strong |
| NSAID133 | Preferably use those with a short half-life such as ibuprofen and paracetamol and administer after each breast feeding | Moderate | Weak |
| Antimalarials130,133 | Hydroxichloroquine and chloroquine | High | Strong |
| Sulfasalazine130,132,133 | Precaution with premature infants, with G6PD deficiency or hyperbilirubinaemia | Moderate | Weak |
| Azathioprine | Precaution in patients with thiopurine methyltransferase deficiency | Moderate | Weak |
| Certolizumab pegola,53,154 | Low responsibility of the doctor | Moderate | Weak |
| Methotrexate and leflunomide130–133 | These are contraindicated | Moderate | Strong |
| Fertility | ||
|---|---|---|
| Recommendations and references | Quality of evidence | Strength of recommendation |
| • Evaluate gestational desire from diagnosis of RA and regularly | Low | Good clinical practice |
| • Offer preconception counselling about fertility, attitude to unplanned pregnancy and preconception planning with non-teratogenic drugs155–157 | Low | Good clinical practice |
| • Recommend control of disease before conception | Low | Good clinical practice |
| • Avoid the use of NSAIDs | Low | Good clinical practice |
In pregnant patients with moderate to high disease activity and in cases of lactation, the panel discussed and concluded that caution is necessary with the administration of certolizumab pegol, that studies showing little bioavailability in the newborn and in milk do not guarantee the absence of toxicity, that the risk benefit should be assessed and the possibility of infection should be carefully monitored.
Infection constitutes one of the main causes of morbidity and mortality in RA patients, and some can be prevented with vaccination. Vaccination coverage in patients with RA is estimated as suboptimal and the role of the rheumatologist in promoting vaccination is stressed, preferably before starting immunosuppressive therapy.120Table 3 describes the main recommendations on vaccination for patients with RA.
Vaccination in patients with RA.
| Vaccine | Recommendation | Quality of evidence | Strength of recommendation |
|---|---|---|---|
| All | When possible, administer in the stable phase of the disease or prior to drug therapy | Low | Weak |
| Influenza24,158 (annual in specified season) | Use is recommended before the season. Tetravalent use is preferred to trivalent. The live virus vaccine (through inhalation) is contraindicated.In the case of the use of methotrexate discontinuation is recommended 2 weeks prior to vaccination.In the case of rituximab an interval between vaccination and the biological drug of at least 6 months is recommended.High dose vaccine for people over 65 | Low | Weak |
| Pneumococcus158 | PCV 13 (few studies as yet). Use single dose preferably prior to starting immunosuppressant treatment, followed by PPV23 one dose 8 weeks after the initial dose of PCV13, and one more dose of PPV23 5 years after the first dose of PPV23161 | Low | Weak |
| Herpes zoster75,159–163(attenuated live virus) | Apply before synthetic DMARD therapy. It is suggested it should be applied 2 weeks prior to starting biological therapy and 4 weeks before JAK inhibitors | Low | Weak |
| HPV | It is suggested that it should be given to women under the age of 26 and men under the age of 21 | Low | Weak |
| Hepatitis B | It is suggested that it should be applied for patients with RA with negative surface antigen and those with risk factors for acquiring hepatitis B | Low | Weak |
| Tdap (tetanus, diphtheria, pertussis) | Single dose for those aged over 19 years who have not received the vaccine | Low | Weak |
Good clinical practice recommendations on screening for TB are based on the TBNET study,121 which is limited to intradermal reaction positivity or Interferon Gamma Release Assay (IGRA) in patients with RA; however, TB was not sought in positive cases. The Enzyme-Linked ImmunoSorbent Assay (ELISA) and the Enzyme-Linked Immunosorbent Spot (ELISPOT) are recommended due to supposed greater sensitivity and specificity compared to tuberculin in patients who have previously received BCG.122
IGRA is not readily available in Mexico, and is also expensive as a screening test. Sensitivity for latent TB in general is similar for PPD and IGRA. In the case of active TB the use of IGRA is recommended.123
In case of PPD (≥5mm) or IGRA positivity, the use of prophylactic therapy with isoniazid is recommended for 9 months.62 However, a recent study showed that the use of rifampicin for 4 months was not inferior in efficacy to the use of isoniazid for 9 months.63
Additional considerationsDue to the prevalence of TB in Mexico and the low availability of IGRA as a screening test, starting prophylaxis of latent TB in patients with RA treated with BioDMARDs or JAK inhibitors with positive PPD is recommended.
Bioethical aspectsIn the recommendations issued, priority is given to the individual needs and preferences of each patient, prioritising the principle of autonomy.124 In the context of chronic diseases such as RA, the patient not only authorised but, together with the medical team, becomes a fundamental part of the execution of the treatment plan (decision autonomy).125
On the other hand, it is necessary to recognise the impact of health disparities on the outcomes of RA patients. In particular, we refer to the lack of distributive justice,125,126 and one of the dimensions of this problem is non-existent or limited access to high-cost drugs such as the BioDMARDs and JAK inhibitors. Bioethical reflection in this regard must address two aspects. The first is the decision (or otherwise) only to include ideal (albeit unreal) clinical scenarios in the current treatment guidelines in which total health coverage is assumed, with the purpose of flagging up the path to follow to the relevant authorities. However, we should not overlook the fact that bioethics must also give specific answers to real situations and not be limited to ethics of unattainable maximums127; in this regard, we as rheumatologists have learned that the early identification of patients with RA and treatment with csDMARDs favourably changes outcomes of patients; implies greater effort and dedication, but implementation is feasible, necessary and ethically justified, since in many clinical contexts it can even overcome the lack of high-cost drugs. The second aspect, linked to the previous one, concerns the effort we must make to raise the awareness of the relevant authorities and include RA as a disease with catastrophic expenditure.128
Finally, possible conflicts of interest have not gone unnoticed in the planning and development of this document (https://es.unesco.org/). Many of the authors were selected based on their experience in the subject, which possibly implies participation and authorship with some of the assessed therapeutic options or with certain reviewed papers. We consider that this experience is valuable, but there is a potential conflict of interest that we declare in this article.
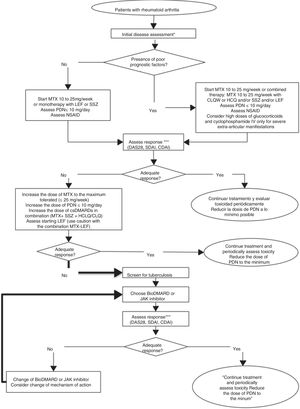
ConclusionsAlthough there are different updated therapeutic guidelines for the treatment of RA, it is very important that the recommendations issued consider the health system that they address. The CMR has updated their treatment recommendations for RA, as well as the treatment algorithm (Fig. 1). The main contributions of this update are as follows: the recommendations of BioDMARDs and JAK inhibitors were combined in the same treatment line, information on pregnancy, lactation, fertility and vaccination is included in the text and the management algorithm was updated. This update was prepared for aspirational purposes and highlights the importance of keeping at least some of these high-cost drugs available in the different health subsystems.
Rheumatoid arthritis treatment algorithm. NSAID: nonsteroidal anti-inflammatory drug; CDAI: Clinical Disease Activity Index; CQL: Chloroquine; DAS28: Disease Activity Score 28; csDMARD: Conventional synthetic disease-modifying antirheumatic drug; BioDMARD: biological disease-modifying antirheumatic drug; HCQ: hydroxychloroquine; LEF: leflunomide; MTX: methotrexate; PDN: prednisone; SDAI: Simplified Disease Activity Index; hidroxychloroquine; LEF: leflunomide. * Initial clinical, serological and radiological assessment of the disease. ** Factors of poor prognosis: seropositivity, erosive disease, high level of clinical activity and extra-articular manifestations. *** Assessment of clinical response can be from 4 to 12 weeks in the case of active disease, every 12 weeks in the case of low disease activity and up to every 6 months in the case of sustained remission, without neglecting pharmacovigilance. Clinical remission or a low level of activity is considered adequate response.
Appropriate treatment of RA should include more efficient systems and processes, with an optimal number of committed rheumatologists, with sufficient time to provide adequate care, as well as pharmacological options that enable good control of the disease. None of the above can be achieved without government support and the commitment of all participants.
FinancingThis project was funded by the Mexican College of Rheumatology AC. The Mexican College of Rheumatology thanks Lilly, Roche, Sanofi and UCP for their financial support through an unrestricted educational fund.
Conflict of interestsMario H. Cardiel declares that he has been a principal investigator, consultant and speaker for Abbvie, Astellas, Eli-Lilly, Janssen, Gilead, Pfizer, Roche.
Sandra M. Carrillo-Vázquez declares that she has been a speaker with Roche, Eli Lilly, Pfizer, Janssen, Novartis, Abbvie, Bristol. She has been sponsored to attend congresses in the past 3 years by Roche, Pfizer, Novartis, Asofarma, UCB, and has collaborated in research protocols with Genentech, GSK, Pfizer, SunPharma, Mallinkrodt ARD, Anthera Pharmaceuticals, Celgene.
Leonardo Limón-Camacho declares having collaborated with Pfizer, Lilly, BMS, Roche, Janssen, UCB, Abbvie, Amgen and Novartis. Sergio R. Gutiérrez Ureña declares having been a consultant, speaker and having received sponsorship for academic events from Pfizer, Lilly, Abbvie, Amgen, Takeda. Sergio Duran-Barragán declares having collaborated with: AMGEN, BMS, Eli Lilly, Glaxo, Merck, Pfizer, Mallinckrodt, Amylin, R-Pharm Speaker: BMS, Roche, Eli Lilly.
Lilia Andrade Ortega declares having received sponsorship from Bristol Myers Squibb, Celgene, Janssen, Novartis, Pfizer, Roche and UCB.
Sandra Araceli Sicsik Ayala declares having been a speaker for Roche, UCB, Pfizer, Bristol, Novartis, Abbvie y Probiomed. She received sponsorship as an investigator from Abbvie, Eli Lily and GlaxoSmithKline.
Leonor Barile Fabris declares being a speaker and consultant for UCB, Pfizer, Abbvie, Roche, Celgene.
María Azucena Ramos Sánchez declares having received sponsorship from Abbvie Farmacéutica, Novartis, Pfizer, Roche and UCB.
Daniel Octavio Grageda Portes declares being a speaker for Roche.
Margarita Portela Hernández declares having received sponsorship from Roche, Pfizer, Bristol, UCB, Abbvie, Janssen and Lily.
José Luis García-Figueroa declares having been sponsored by Expanscience and Bristol Myers Squibb.
Cesar Pacheco-Tena declares having collaborated with Abbvie, Roche, Bristol-Myers-Squibb, UCB, Merck-Serono, Astra-Zeneca, Novartis, Eli-Lilly, R-Pharm, Actelion, Sanofi, Janssen, Pfizer and Sandoz.
Mauricio Montero Luna declares having been sponsored for consultancy, academic events and conferences organised by Novartis, Bristol-Myers-Squibb, Roche, Pfizer, UCB, Abbvie, Janssen, Expanscience and MSD.
Fedra Consuelo Irazoque Palazuelos declares having been sponsored by Roche, Novartis, Celgene, Takeda, Pfizer, Lily, Janssen, Expanscience, Sanofi and Abbvie.
Carlos Abud-Mendoza declares having participated as a consultant and speaker for Pfizer BMS, UCB, Lilly and Roche.
Virginia Pascual Ramos declares having been an investigator in projects of Pfizer and with UCB.
Sergio Cerpa Cruz declares having participated as an investigator for Sanofi, Astra Zeneca and Neovacs.
Jorge Enrique Aguilar Arreola declares having participated as a speaker for Roche and Pfizer and having received support to attend congresses of Pfizer, Roche, Lilly, Abbvie and Asofarma.
Alina Hernández-Bedolla declares having received a grant for congresses for Bristol and ABBVIE, and being a conference speaker for Roche.
Luis H. Silveira declares having received a grant from Novartis to attend the two last Mexican Rheumatology congresses.
Alejandra López Rodríguez declares having participated as a speaker for Roche, Eli Lilly, Pfizer, Celgene, BMS and in clinical research with Pfizer, Novartis, Eli Lily, Glaxo; she is also a consultant with: Roche, Novartis, UCB, Eli Lily.
Greta Reyes-Cordero declares a conflict of interest with Pfizer, UCB, Novartis, Bristol-Myers Squibb, Roche.
Humberto Alfredo Ricardez Puente declares a conflict of interest with Bristol-Myers Squibb as a subinvestigator and for attending congresses and/or updates for Abbive, Pfizer, Janssen, Lilly.
María Fernanda Hernández Cabrera declares a conflict of interests with Roche and Abbvie.
Javier Merayo-Chalico was a speaker for Pfizer and was sponsored to attend two academic events.
Daniel Xibillé Friedmann declares having been a principal investigator, consultant and speaker for Abbvie, BMS, Eli-Lilly, Janssen, Pfizer, Roche.
Sandra Muñoz López, Istar Guzmán Sánchez, María Esther Pérez-Bastidas, Marco Ulises Martínez Martínez, David Herrera van Ostdam, Guadalupe Olvera-Soto and Marcela Pérez have no conflict of interest to declare.
The Mexican College of Rheumatology would like to acknowledge the valuable contributions and suggestions of Concepción Cazariego from AMEPAR, Dr. Cecilia Guerrero Almeida, Dr. Polita del Rocío Cruz Cruz, Dr. Sara Morales Hernández and Dr. Alejandro E. Macías, who in their role as patients, infectologists and obstetrician-gynaecologists improved the content of our guidelines.
Please cite this article as: Cardiel MH, Carrillo S, Pérez M, Andrade L, Pacheco Tena C, Silveira LH, et al. Actualización de las guías del tratamiento farmacológico de la artritis reumatoide del Colegio Mexicano de Reumatología 2018. Reumatol Clin. 2021;17:215–228.
The constant therapeutic advances in rheumatoid arthritis (RA) make it necessary to periodically review treatment guidelines in order to offer the treating physician updated, evidence-based recommendations adapted to real clinical practice.