Ultrasound is a non-invasive, innocuous, reproducible, cost-efficient imaging technique that provides immediate information, as it can be performed in our consultations. The good profile of ultrasound and the technological advances that have taken place in recent years, which have allowed a substantial improvement in the resolution of the image to make it almost anatomical, have promoted research on its application in the comprehensive study of systemic inflammatory diseases. At present, the threshold of using ultrasound to exclusively investigate musculoskeletal manifestations has been crossed, to also apply it to the study of extra-articular manifestations and comorbidities associated with rheumatic diseases. In this review we will revise its usefulness for the diagnosis of glandular involvement in Sjögren's syndrome, interstitial lung disease or giant cell arteritis and for stratification of cardiovascular risk in patients with chronic inflammatory rheumatic diseases.
La ecografía es una técnica de imagen no invasiva, inocua, reproducible y coste-eficiente, que aporta información inmediata al poder realizarla en nuestras consultas. El buen perfil de la técnica y los avances tecnológicos acontecidos en los últimos años, que han permitido una mejora sustancial en la resolución de la imagen hasta hacerla casi anatómica, han impulsado la investigación sobre su aplicación al estudio integral de las enfermedades inflamatorias sistémicas. En la actualidad, se ha traspasado el umbral de utilizar la ecografía para investigar exclusivamente las manifestaciones músculo-esqueléticas, para aplicarla también al estudio de las manifestaciones extra-articulares y las comorbilidades asociadas a las enfermedades reumáticas. En la presente revisión repasaremos su utilidad para el diagnóstico de la afectación glandular en el síndrome de Sjögren, la enfermedad pulmonar intersticial o la arteritis de células gigantes y para la estratificación del riesgo cardiovascular en pacientes con enfermedades reumáticas inflamatorias crónicas.
The inflammatory joint diseases, the paradigm of which in terms of prevalence and progress in knowledge is rheumatoid arthritis (RA), may present extra-articular manifestations and/or comorbidities associated with the inflammatory process during their evolutionary process, even during the early phases of the disease or as its first manifestation. These associated process not only affect patient quality of life negatively, as they may also sometimes compromise the working of an organ or life expectancy. In systemic vasculitis such as giant cell arteritis (GCA), visual compromise in the predominantly cranial forms or the involvement of large vessels that may threaten life or organs, means that early diagnosis is indispensable.
Ultrasound scan is harmless and is tolerated well by patients. This, together with research work and technological progress over recent years means that this technique is now one of the first-line diagnostic techniques for the early detection of certain extra-articular manifestations and comorbidities such as cardiovascular risk (CVR) in patients with systemic inflammatory diseases. In this review we centre on the most relevant and useful aspects for diagnostic use in everyday clinical practice.
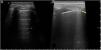
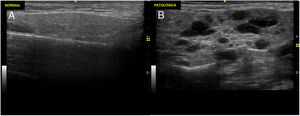
Ultrasound scan of the large salivary glands to diagnose Sjögren's syndromeThe ultrasound scan of large salivary glands (EcoGS), parotid glands and submandibular glands, has been proven to be able to detect typical structural alterations in the glandular parenchyma of patients with primary Sjögren's syndrome (SSp). These patients have more frequent and severe involvement than healthy controls or other connective tissue pathologies.1,2Fig. 1 shows example of normal parotid gland ultrasound scans and others of Sjögren's syndrome. Although no clearly differentiating ultrasound findings have been identified between SSp and other connective tissue pathologies, a potential and predominant involvement has been described in the submandibular gland versus the parotid glands in the latter, unlike the frequent involvement of both types of gland in SSp.2
Ultrasound scan image of normal parotid and one of a patient with Sjögren's syndrome.
Ultrasound scan images showing parotid glands with normal and pathological parenchyma. A). Ultrasound image of a normal parotid. B). Changes marked grade 3 according to the OMERACT index: diffuse loss of parenchymal homogeneity with hypo/anechoic areas that occupy the whole glandular surface, without normal tissue.
In terms of its diagnostic efficacy EcoGS has been shown to be comparable to other techniques used, such as syalography and salivary gammagraphy, and it is very close to magnetic resonance imaging (MRI). Although the latter has been shown to have slightly better sensitivity and specificity than EcoGS in parotid gland evaluation, the lower costs and higher degree of availability of ultrasound make it preferential for use in everyday clinical practice.3
Respecting the relationship between EcoGS and the smaller salivary gland (BioGS), studies which directly compared both techniques showed a moderate degree of correlation (r=0.412) in a cohort of 103 patients with the suspicion of SSp, in which EcoGS (parotid) was compared with parotid biopsy and BioGS;4 and good (r=.61; P< .01), in a sub-study of the TEARS trial, in the 24 patients in which both techniques were performed.5
The role of EcoGS in the classification of SSp has been studied by evaluating the impact of adding it as an additional item in different classification criteria, including the recent ACR-EULAR6 criteria, estimating a weighting of pathological ultrasound scan of 1, which is equivalent to its minor criteria. In this context and without modifying the cut-off point for classification, EcoGS achieves an increase in sensitivity (97.3% vs. 95.9%) with a minimum reduction in specificity (92.2% to 90.2%). Nevertheless, its use as a substitute for items in these criteria is more controversial. While the substitution of minor items by EcoGS does not seem to affect the performance of criteria, it is not considered advisable to use it to substitute major items (BioGS or anti-Ro antibodies) as it substantially reduces their sensitivity.6
The main limitation for the use of EcoGS in clinical practice to date has been underlined by the high level of heterogeneity between studies, especially regarding the definition of lesions and indexes, together with reliability data that are not fully robust. To overcome these limitations, the EULAR Sjögren group has identified the elemental lesions for which it is most reliable in terms of the homogeneity and echogenicity of the glandular parenchyma. They did so for static as well as acquired images7 and the elements that contribute independently to prediction of classification by the ACR-EULAR criteria, echogenicity and the presence of hypo- anechoic foci in parenchyma (AUC=0.857; R2 = 0.539).8 The strong correlation that exists between echogenicity and hypo-anechoic areas, and between the glandular findings on each side, unlike interglandular variability, makes it possible to restrict ultrasound scan evaluation to the study of hypo/anechoic areas in a parotid gland and a submandibular gland, maintaining precision (AUC=.846; R2 = .498) while increasing the feasibility of examination.8
Regarding these premises, after three Delphi rounds a broad international panel of experts agreed the definitions of echographic normality and elemental lesions, together with the examination protocol and a semiquantitative echographic index (0-3) following OMERACT norms. This includes the possibility of applying a qualitative evaluation of a gland with fatty or fibrous degeneration when this is not quantifiable (Table 1). The degree of reliability, following the evaluation of video recordings in a web platform, was excellent at intraobserver level and good in interobserver terms (Light’s kappa 0.79 and 0.62, respectively).9
OMERACT group ultrasound index of the larger salivary glands (parotid and submandibular).
| Items evaluated | Ultrasound findings | Score |
|---|---|---|
| Semiquantitative index | ||
| 0 = normal | Normal parenchyma | 0 |
| 1 = minimum changes | Slight loss of parenchymal homogeneity with no hypo- anechoic areas | 1 |
| 2 = moderate changes | Moderate loss of parenchymal homogeneity with focal hypo- anechoic areas surrounded by normal tissue | 2 |
| 3 = marked changes | Diffuse loss of parenchymal homogeneity with hypo - anechoic areas that occupy the whole glandular surface, with no normal tissue | 3 |
| Qualitative index | ||
| Fatty gland | Gland diffusely hyperechogenic in comparison with the surround soft tissues | 1 |
| Fibrous gland | Fibrotic gland with hyperechoic glandular bands that make it indistinguishable from the surrounding soft tissues | 3 |
OMERACT: Outcome Measures in Rheumatology.
In an attempt to improve the reliability and feasibility of EcoGS, with the aim of making it an effective and non-invasive diagnostic tool that could replace current diagnostic tests, the European Union Project HarmonicSS is subjecting ultrasound scan images to an automatic process of segmentation and analysis using artificial intelligence algorithms. A preliminary analysis of 600 EcoGS, obtained and scored by expert ultrasound scan users in two SSp cohorts, has identified the MLP (Multilayer perceptron) algorithm as the best classifier (κ=.7), with an intraobserver reliability within human range (κ=0.71) surpassing average human interobserver reliability (κ=.67).10 These advances may become more important as the size of the final cohort increases.
The time taken to examine the larger salivary glands by ultrasound scan evaluation is described as varying from 11 to 27minutes for the most extensive assessments in terms of their parameters and the number of glands to be examined.7 However, the new echographic index proposed by OMERACT9 increases its feasibility by reducing the time taken for examination by half.
Ultrasound scan for the study of diffuse interstitial pulmonary diseaseThe delay in the application of pulmonary ultrasound scan (EcoPulm) in Rheumatology has been due to its being restricted by acoustic barriers, given that air and bone do not conduct ultrasound, and because this would involve a conceptual shift. It is based on interpretation of the findings associated with changes in the physical properties of the lung, which are artefacts rather than anatomical, which we have always tried to avoid in musculoskeletal ultrasound scans.
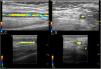
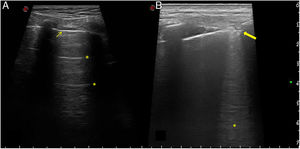
Studies of the usefulness of EcoPulm in screening for diffuse interstitial pulmonary disease (DIPD) have centred on evaluating B lines and the pleural line (Table 2). The B lines or "comet-tails" are artefacts which originate when pulmonary parenchyma air content partially falls and/or the interstitial space expands volumetrically. They do not give an etiological diagnosis, as they also occur in other pathologies such as oedema, and nor do they permit discrimination between inflammatory or fibrotic phases of DIPD. The pathological pleural line may show irregularities, thickening, fragmentation and subpleural nodules. Fig. 2 shows ultrasound scan images of healthy lung and lung affected by DIPD.
Pulmonary ultrasound scan patterns showing normality or diffuse interstitial pulmonary disease.
| Types of pattern | Ultrasound scan findings | Description and origin of artefacts |
|---|---|---|
| Normal lung pattern | Fine and regular pleural line | Horizontal hypoechoic line visible between costal edges, located 0.5cm below. Produced by the pleuro-pulmonary interface |
| Pulmonary slip | An oscillating motion of the pleural line, synchronised with respiration. It corresponds to pulmonary slip along its craniocaudal axis | |
| A lines | Horizontal hyperechoic lines located under the pleural line at regular intervals. They represent intercostal musculature reverberation artefacts | |
| Diffuse interstitial pulmonary disease pattern | B lines | Vertical clearly defined hyperechoic lines of the laser beam type, emerging from the pleural line and extending into the depth without fading, erasing the A lines. Deriving from the thickening of the subpleural interlobular septi |
| Alterations of the pleural line | Irregularity, thickening, fragmentation, subpleural nodules |
Normal lung ultrasound scan and with interstitial pulmonary disease.
Ultrasound scan images showing normal and pathological lungs. A). Normal lung pattern. A fine and regular pleural line can be seen (thin arrow), visible between the costal edges, which leave a posterior shadow, as well as the presence of A lines (asterisks), which are the horizontal hypoechoic lines located under the pleural line at regular intervals. B). Pathological lung pattern. The pleural line shows irregularity, thickening and fragmentation (thick arrow). B lines can be seen (asterisk), which are the hypoechoic vertical lines running from the pleural line to the depth without fading, erasing the A lines.
The most solid evidence has been published for scleroderma, where evaluation of the validity of the appearance, criterion and construct is more advanced for evolved as well as earlier stages of the disease. Thus a good correlation has been described between B lines and other diagnostic techniques for DIPD such as high resolution axial computed tomography (HRACT) (r=.81; P< .0001) and respiratory function test parameters such as lung capacity to diffuse carbon monoxide (DLCO) (r = -.60; P< .05) and forced vital capacity (FVC) (r = -0.48; P< .001).11,12 Its correlation has also been described with clinical-analytical parameters such as antitopoisomerase-1 (Scl70) antibodies, the diffuse cutaneous involvement sub-type, damage progression in capillaroscopy, digital ulcers or the severity index.12 Reported precision is high, with high sensitivity values (83.9%) and specificity values (87%) and a diagnostic odds ratio (OR) (42.9)13 and good intra- and interobserver reliability,11 making EcoPulm a potential screening method for DIPD in scleroderma.
Outstanding limitations of the technique include the high level of heterogeneity of the evidence that has been published in terms of B line echographic counts and indexes, the cut-off points set to define disease, the equipment used (from upper range ultrasound scan equipment down to pocket apparatus), as well as the probes used (cardiological, lineal or convex) or examiner experience. After the first 72 intercostal space indexes11 progressively smaller counts have been reported, down to 10 intercostal spaces,14 to increase feasibility while attempting to maintain precision. Nevertheless, there is still no agreement on the learning curve or the procedure, and nor has any index been validated that would facilitate its implementation in clinical practice with the necessary guarantees.
Respecting pleural line evaluation, some authors suggest that it has greater negative predicative value (NPV) for DIPD than the B lines, together with greater discrimination against healthy controls, as while B lines are present in 35% of patients with scleroderma who do not show any signs of DIPD in HRACT and in 7% of controls, no alterations are found in the pleural line in either of these two contexts.15
In other diseases such as SSp, RA or antisynthetase syndrome studies are at a preliminary phase and are restricted by small sample size. However, the results seem to be along the same line as those for scleroderma.16–18
Although the data are promising, at the current time we do not have evidence that would allow us to use EcoPulm as an alternative to conventional diagnostic tests or to replace them in follow-up, so that we await the outcome of completion of its validation.
Respecting the time necessary to examine and interpret pulmonary ultrasound scan imaging, this will depend on the index used. It will vary from 5-6minutes for the shortest times to 9minutes for abbreviated use and 23-24minutes for the most extensive studies.11,14
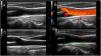
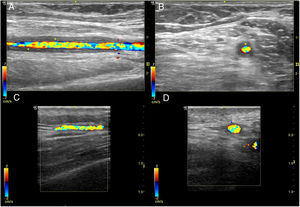
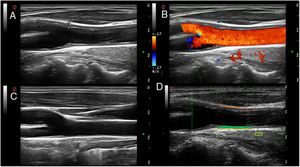
Ultrasound scan of temporal and axillary arteries for the study of GCAEvidence for the utility of ultrasound scan imaging for the diagnosis of GCA has accumulated over the past 20 years, and its validity has been confirmed in several meta-analyses. Due to this, the recent EULAR recommendations on the use of imaging tests in large vessel vasculitis in clinical practice19 define ultrasound scan imaging of the temporal and/or axillary arteries as the first imaging technique recommended for patients with the suspicion of predominantly cranial GCA, considering the non-compressible "halo sign" to be the most relevant finding. The positive compression sign is characterised by the persistence of vascular wall visibility (the halo) when pressure is exerted by the probe on its vasculitic thickening. It is simple, fast and reliable (interobserver Krippendorff’s α .92) with high levels of sensitivity (75%-79%) and specificity (100%) for the diagnosis of GCA.20Fig. 3 shows the characteristic halo sign in the ultrasound scan of a patient with GCA, in comparison with an ultrasound scan image of a normal temporal artery.
Normal temporal artery ultrasound scan image and one with giant cell arteritis.
Temporal artery halo sign in a longitudinal slice (A) and a transversal slice (B). Thickening of the temporal artery wall is visible (asterisks). Images C and D show ultrasound longitudinal and transversal images of normal temporal artery, respectively.
The preference for ultrasound scan imaging, on condition that it is swiftly available and the expertise of the examiner is guaranteed, for temporal artery biopsy (TAB) is due to the fact that it is less invasive, gives immediate results with a high level of evidence, and it is also less expensive.19 The choice of the temporal and axillary arteries for routine examination in case of the suspicion of GCA is due to the fact that they have always shown a halo in addition to vasculitic findings in other vascular areas when conducting extensive evaluations of the major peripheral vessels, and because less time is required for examination, increasing its suitability for clinical practice.21
The start of treatment with steroids should never be delayed due to an imaging test, and it is preferable to do this in the first week. This is because the average time to resolution of the halo is usually from two to three weeks, and it takes longer if a larger number of branches are affected.22 Some authors describe a faster reduction in sensitivity after five days.19
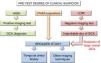
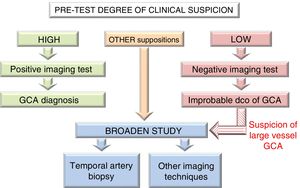
The need to use additional diagnostic techniques such as TAB or other imaging techniques (MRI, CT and PET-CT), or to broaden ultrasound scan examination to include other vascular territories, will be determined by the degree of pre-test clinical suspicion and the results of the first imaging technique used. Thus if suspicion is high and the image is positive, or if clinical suspicion is low and the image is negative, GCA diagnosis may be performed or excluded, respectively, without the need for additional tests. In other situations or if there is the suspicion of extracranial arteritis, it is recommended that the study be broadened19 (Fig. 4).
Giant cell arteritis diagnostic algorithm.
Algorithm applicable to hospitals where ultrasound scans can be performed early and with suitable quality. Nevertheless, in hospitals where ultrasound scan may not be available early or when the expertise of the examiner is questionable, priority will be given to temporal artery biopsy as the first diagnostic test.
GCA: Giant Cell Arteritis; DCO: Diagnosis.
The clinical impact of using ultrasound scan imaging for diagnostic purposes in the context of rapid referral circuits has been studied, comparing the outcomes obtained to those corresponding to conventional clinical practice, as well as in historic cohorts. The benefit is clear as it significantly reduces the time of evolution until diagnosis (79% in 24hours vs. 64.6%, P= .023) and permanent sight loss (9% vs. 37%), in a fast circuit versus conventional clinical practice, respectively.23
Progress is being made towards being able to measure the intima-media thickness (IMT) of the different vascular territories affected by an earlier and more exact diagnosis of GCA, for which upper range ultrasound scan equipment is required. IMT cut-off points have been identified which are associated with vasculitic involvement: .29-.42mm for the different branches of the temporal arteries and 1mm for the axillary arteries.24
On the other hand, it would seem to be advisable to perform a complete evaluation of these patients, as from 12% to 50% of them will have extracranial involvement21 and imaging techniques can help us to identify patient clinical subtypes, depending on whether they have temporal artery and extracranial involvement. In patients with exclusively temporal artery involvement visual and ischemic symptoms predominate, while those with mainly extracranial involvement, the predominant form of the disease in women, younger patients with few visual symptoms and cranial ischemia, tend to have more pulmonary and vascular symptoms in the upper and lower limbs. Combined involvement is more frequent in older men, with major analytical alterations and cranial and vascular symptoms, above all in the lower limbs.25
Lastly, in 2011 an international observational study commenced under the auspices of EULAR and ACR: the DCVAS (Diagnostic and Classification Criteria for Vasculitis). It is designed to develop and validate diagnostic criteria, as well as to improve and validate the classification criteria of the six forms of systemic vasculitis, including GCA.26 The study covers clinical, analytical, biopsy and imaging data, including ultrasound scan. A preliminary proposal was presented in the form of an oral communication in the 2018 ACR Congress, although they are still pending validation and publication.
Little time is taken to detect vasculitic changes in the temporal arteries using the sign of positive compression, at 6.4±2.1minutes.20 This may increase to about 10 to 15minutes if it is also necessary to evaluate the axillary arteries.
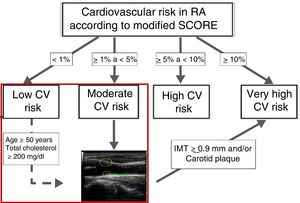
Carotid ultrasound scan for the stratification of CVRRA and other immunologically-mediated chronic inflammatory diseases are strongly associated with atherosclerosis, and they intervene in this process in terms of disease characteristics as well as in traditional cardiovascular risk factors (CVRF). This is why, to take the importance of inflammation into account, recent EULAR recommendations for the management of CVR in these patients advise using a modified SCORE (Systematic Coronary Risk Evaluation) (mSCORE) that includes a 1.5 multiplier factor in all patients with AR.27
As well as the SCORE and the Framingham index28 (which is used more in the United States) other systems are used to predict CVR. These predictive models differ in terms of the CVRF they include, as well as in their thresholds and therapeutic goals. We have no evidence that indicate any one of these is superior to any of the others, as they perform in a similar way when applied to populations with similar characteristics to those of the ones their calculation is based on. Due to this last reason the European guides for the prevention of cardiovascular disease29 and the EULAR recommendations for the management of CVR in rheumatic diseases27 advise the use of the SCORE in Europe, as this system is based on data obtained in 12 representative European cohorts. It has also been recalibrated in some countries where CVR is low (including Spain) and has been externally validated, making it more interesting for clinical practice.
However, the available evidence from a population-based study in patients with RA in our environment and without diabetes mellitus, kidney disease or other CVRF, indicate that this mSCORE underestimates CVR, giving a sensitivity of 18% (CI 95%, 12-25). While the mSCORE only reclassifies risk to high or very high for five of the 327 RA studied, the finding of plaques and/or increased carotid IMT (cIMT) in ultrasound scan of the carotid (EcoCar) reclassifies risk to very high in 13% of the patients classified as low risk and 63% of those classified as moderate risk.30 In a subsequent study centred exclusively on RA with low risk on the mSCORE, the multivariant regression models to predict the presence of plaque identified age and total cholesterol concentration in the serum as predictive factors, with an optimum cut-off point of 50 years old for age and 200mg/dL for cholesterol.31 With these items of evidence it would be possible to update a previous algorithm for CVR stratification in RA that includes EcoCar.32 This would recommend it for the evaluation of patients at moderate risk according to their mSCORE and in selected low-risk cases. The patients with a cIMT ≥ .9mm or carotid plaque will be reclassified with a very high CVR (Fig. 5).
Rheumatoid arthritis cardiovascular risk stratification algorithm.
The ultrasound image shows carotid plaques framed by circles. The intima-media thickness corresponds to the area shaded in green between the intima and the interruption in the far wall of the carotid.
CV: Cardiovascular; IMT: Intima-media Thickness; RA: Rheumatoid Arthritis; SCORE: Index for Systematic Coronary Risk Evaluation.
Modified by: González-Gay MA. Ann Rheum Dis. 201232.
For other chronic inflammatory diseases of the joints we found comparable data for psoriatic arthritis, with an increase in the prevalence of plaques and high cIMT, as well as greater CVR-reclassification capacity in EcoCar in comparison with mSCORE.33
Plaque is a recognised cardiovascular disease marker, and it is also a modifying factor of CVR classification in all prevention guides, including those of EULAR which recommend screening for asymptomatic atherosclerotic plaques using EcoCar in patients with AR.27 However, the value of cIMT is highly controversial, as use of the same has been said to be unadvisable to evaluate the risk of a first cardiovascular event in clinical practice by the most recent guides for evaluation of CVR of the American College of Cardiologists and the American Heart Association, as well as those of the European Guides for the Prevention of Cardiovascular Disease in Clinical Practice. Longitudinal population studies are currently underway to help clarify this crucial aspect. Fig. 6 shows ultrasound scan images of the normal carotid and ones with plaques, together with radiofrequency measurement of cIMT.
Ultrasound scan of normal carotid and one with plaque, and measurement of cIMT.
Images A and B show a carotid artery with soft atheroma plaques (asterisk) in the carotid bifurcation, in a scale of greys (delimited by measurement symbols "+") and Doppler in colour, respectively. The atheroma plaques are shown as focal protrusions in the vascular span. In the example shown in image A the plaque measures 1.7mm from the media-interruption interface to the intima-l vascular span interface. Image C shows a normal carotid. Image D shows radiofrequency measurement of the carotid cIMT. The cIMT is represented by the green area between the intima- vascular span interface and the media-interruption interface (orange line) in the far wall of the carotid.
Regarding the utility of implementing T2T strategies in clinical practice in comparison with classic CVRF in RA, the results of one recently published study associate this strategy with a significant reduction in the progression of cIMT, LDL cholesterol levels in serum and fatal and non-fatal cardiovascular events after five years of follow-up compared with habitual clinical practice.34 These results suggest that rheumatologists should play a more proactive role in the management of CVR for their patients, most of all when the time taken to evaluate the presence of plaques and the measurement of cIMT amounts to from 10 to 15minutes, depending on the skill of the examiner.
To summarise, EcoGS seems to increase the sensitivity of SSp Classification Criteria. The evaluation of B lines and the pleural line is promising for DIPD screening. Temporal and/or axillary artery ultrasound scan is recommended as the first imaging test in case of suspicion of GCA. Carotid ultrasound scan improves the stratification of CVR in rheumatoid arthritis, and it may also do so in other chronic inflammatory diseases.
The agenda for future research into the use of ultrasound scan in the study of extra-articular manifestations of systemic rheumatic inflammatory diseases is very broad and exciting. In the diagnostic field it is necessary to complete the process of agreeing feasible ultrasound imaging indexes that can be included as added value elements to the diagnostic and classification criteria of the diseases in question. Likewise, it is necessary to continue researching the role of ultrasound scan as a potential biomarker of subclinical activity and therapeutic response, especially given the arrival of highly effective biological treatments that are also highly expensive and not free of side effects. Another highly interesting area is the one in connection with role of ultrasound scan as a tool to evaluate damage and follow-up patients, for which multicentre studies will be required with a suitable sample size that makes it possible to draw conclusions that are applicable to clinical practice. Lastly, advances in the field of the application of artificial intelligence to the evaluation of examinations using imaging techniques may lead to a qualitative leap that improves the diagnostic process for these diseases. Nor should we forget that new fields of interesting are emerging, such as skin evaluation in diseases such as scleroderma or psoriatic disease. All of this creates major challenges that have to be faced. The first one of these is technological, as it will be necessary to use upper range ultrasound equipment that includes different types of probe (conventional lineal probes as well as convex small footprint ones), which cover a broader range of frequencies (from low to high or very high), to be selected depending on the structure to be examined, and with the aim of obtaining maximum resolution images. On the other hand there is also the human challenge, as it is necessary to establish supervised training programs that guarantee examiner experience and expertise.
FinancingThis research was received no specific financial support from public sector agencies, those in the private sector or not-for-profit bodies.
Conflict of interestsThe authors have no conflict of interests to declare. This review was presented in part as an oral communication in the XXIII Congress of the Society of Rheumatology of the Autonomous Community of Madrid (SORCOM) in Madrid, December 2019.
Please cite this article as: Vicente-Rabaneda EF, Acebes C, Castañeda S. Utilidad de la ecografía extraarticular aplicada a las enfermedades inflamatorias sistémicas en la práctica clínica. Reumatol Clin. 2021;17:229–236.