To compare the short-term efficacy and safety of rituximab (RTX) therapy versus anti-TNF in rheumatoid arthritis (RA) patients after discontinuation of a first anti-TNF agent.
MethodsProspective observational multicenter study in the clinical practice setting, involving patients with severe RA refractory to a first anti-TNF agent, who received either RTX or a second anti-TNF (2TNF), comparing the efficacy endpoints, EULAR response (Good/Moderate) and safety at 6 months.
Results103 patients enrolled, 82 completed 6-month follow-up, 73.7% women. Baseline data for RTX and 2TNF groups, respectively: TJC, 8.6 and 6.6; SJC, 8.8 and 7.5; DAS28 score, 5.45 (±1.28) and 5.18 (±1.21) (P=.048), ESR, 41 and 38.7mmHg; and HAQ, 1.2 and 1.0. Improvement was observed in all parameters, with no significant differences (except for a more marked reduction in ESR with RTX). There were no serious adverse events.
ConclusionsRTX use as second-line therapy after anti-TNF failure led to improvements in the efficacy and functional variables at 6 months, with no serious adverse events. These results were comparable to those observed in patients who used a second anti-TNF agent in the same clinical scenario.
Evaluar la eficacia y la seguridad a corto plazo del tratamiento de pacientes con artritis reumatoide (AR) con rituximab (RTX) comparado con un anti-TNF (2TNF) tras retirada de un primer anti-TNF.
MétodosEstudio multicéntrico prospectivo, observacional, de práctica clínica de pacientes con AR grave refractaria a anti-TNF que recibieron RTX comparados con los que recibieron un 2TNF. Comparación de las variables de eficacia y respuesta EULAR buena/moderada a los 6 meses.
ResultadosCiento tres pacientes incluidos; 82 alcanzan seguimiento a 6 meses, 73,7% mujeres. Datos basales grupo RTX y 2TNF, respectivamente: 8,6 y 6,6 NAD, 8,8 y 7,5 NAI, 5,45 ± 1,28 y 5,18 ± 1,21 en DAS28 (p=0,048), 41 y 38,7mmHg de VSG, y 1,2 y 1,0 en HAQ. Mejoría en todos los parámetros en ambos grupos sin diferencias significativas (excepto mayor reducción de VSG con RTX). Ausencia de efectos adversos graves.
ConclusionesEl uso de RTX en segunda línea de terapia biológica tras fallo a un primer anti-TNF en práctica clínica muestra mejoría en las variables de eficacia y funcionalidad a los 6 meses, sin presentar efectos adversos graves. Estos resultados no difieren de los observados tras el uso de un segundo anti-TNF en el mismo escenario clínico.
Rheumatoid arthritis (RA) is an inflammatory, autoimmune disease, that affects the Spanish population with a prevalence estimated to be 0.5%, and is more common in women than in men. It is a chronic disabling disease with several social and economic costs and a considerable psychological impact, in addition to the substantial decrease in quality of life related to the health of these patients.1 The treatment of RA, since the development of biologic disease-modifying drugs, has advanced in terms of controlling the disease, improving the symptoms and the progression of joint damage, with a good safety profile.2
Given the first appearance of anti-tumor necrosis factor (TNF) agents as a biological therapy (BT), they have been the most widely utilized approach. The recommendations of the Spanish Society of Rheumatology3 endorse both the possible change in therapeutic target, as well as the replacement of an anti-TNF agent for another. It could be that there are patients for whom a change in therapeutic target is more interesting than changing from a TNFα inhibitor over the successive therapeutic lines.
There are data in the scientific literature that support both the change (switching) of an anti-TNF by another, consecutively, as well as a change in the therapeutic target, with rituximab, among others, even making known their preference for the latter among seropositive patients.4–8 Some of these results come from large observational registries, but not from prospective studies.
The objective of our report is to evaluate the clinical efficacy and safety 6 months after beginning treatment with rituximab, compared to a second anti-TNF in the second BT line among patients with a diagnosis of RA, in clinical practice.
Patients, Material and MethodsThis is a prospective observational study in the clinical practice setting, that was postauthorized for clinical practice in patients with severe RA (1987 American Rheumatism Association criteria9) being treated with a second line of BT, after having an inappropriate response or intolerance to a first anti-TNF agent. The study was approved by the ethics committee of each participating center, and the patients gave their informed consent. A total of 9 Spanish centers at the national level and 14 rheumatologists participated in the study, which covered the period from June 2009 to September 2011.
The patients were recruited consecutively and received either a second anti-TNF (2TNF), or rituximab (RTX), depending on the judgment of each researcher and in accordance with the recommendations of the Guidelines concerning the use of biological therapy provided by the Spanish Society of Rheumatology.3
Baseline variables and 6 months later (prior to beginning the second cycle of RTX administration):
- -
Age, sex, duration of the disease, cause for discontinuing the first anti-TNF (primary or secondary, intolerance/adverse effect).
- -
Tender joint count (TJC), swollen joint count (SJC).
- -
Erythrocyte sedimentation rate (ESR) in mmHg, C-reactive protein (CRP) in mg/dL, positive IgM rheumatoid factor (RF) (if titer >15U/mL) and titer by nephelometry, positive anti-citrullinated protein antibodies, if titer >20U/mL, by means of enzyme immunoassay.
- -
Disease Activity Score in 28 joints (DAS28) of 3 variables including ESR.10
- -
Mild adverse effect: does not require hospital admission, is treated by discontinuing the drug; severe adverse effect: requires hospital admission and specific treatment.
- -
Questionnaire in Spanish validated by the Health Assessment Questionnaire (HAQ).11
The purpose was to compare the clinical effects of RTX and those of a 2TNF as a second line of BT in RA patients 6 months, after a good/moderate European League Against Rheumatism (EULAR) response,12 using improvements in ESR, CRP, RF and HAQ. We also evaluated the percentage of patients who request switching the drug because of an adverse effect.
Statistical AnalysisThe statistical analysis will be based on the data introduced into the database, with a descriptive analysis of all the categorical and continuous variables.
To make comparisons over the study period, in the case of continuous variables, the Snedecor F test (ANOVA) is used, if the variables are normally distributed, or the Friedman test if they do not meet parametric criteria. In the case of dichotomous variables, the Cochran test should be utilized.
The value of the statistical significance is established at P<.05. All the analyses are done with the SPSS 19.0 software package.
ResultsWe included a total of 103 patients, 54 in the RTX group and 49 in the group for 2TNF (23 etanercept [47%], 16 adalimumab [32%] and 10 infliximab [20%]); the major sociodemographic and clinical variables of the latter are shown in Table 1.
Baseline Sociodemographic and Clinical Variables.
| Rituximab (n=54) | 2 TNF (n=49) | Total (n=103) | |
|---|---|---|---|
| Age (years) | 58.0±14.1 | 50.9±12.3 | 57.4±13.0 |
| Gender (female, %) | 77.7% | 67.3% | 73.7% |
| Duration of RA (years) | 5.7±3.2 | 6.1±2.9 | 5.8±3.6 |
| DAS28 | 5.45±1.5a | 5.18±1.8a | 5.37±1.7 |
| HAQ | 1.2±0.8 | 1.0±0.7 | 1.1±0.7 |
| TJC | 8.6±4.5 | 6.6±4.1 | |
| SJC | 8.8±4.1 | 7.5±3.7 | |
| ESR (mmHg) | 41.0±24.1 | 38.7±22.9 | 40.5±26.4 |
| CRP (mg/dL) | 27.6±18.4 | 21.4±12.7 | 24.7±15.0 |
| Rheumatoid factor | |||
| Titer (U/mL) | 99.6±80.2 | 82.7±64.7 | 91.2±73.4 |
| % (positive) | 70.2% | 75.7% | 72.8% |
| ACPA (positive) | 50.2% | 53.1% | 51.4% |
| Previous TNF | |||
| Etanercept | 31% | 32% | 33% |
| Infliximab | 25% | 22% | 26% |
| Adalimumab | 44% | 46% | 41% |
| Reason for discontinuing previous TNF | |||
| -Primary inefficacy | 93% | 94% | 93% |
| Secondary inefficacy | 5% | 0% | 4% |
| Adverse effect and/or intolerance | 2% | 6% | 3% |
| Concomitant use of methotrexate | 92.5% | 77.5% | 87.3% |
Results expressed as mean±standard deviation and percentages.
ACPA, anti-citrullinated protein antibodies; CRP, C-reactive protein; DAS28, Disease Activity Score 28 joints; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; RA, rheumatoid arthritis; SJC, swollen joint count; TJC, tender joint count; TNF, anti-tumor necrosis factor α.
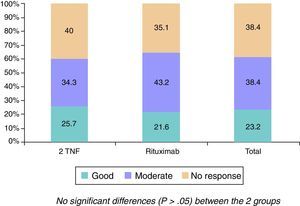
The changes in the clinical, serological and functional parameters are represented in Table 2. There was a significant reduction in all the clinical, serological and functional variables at 6 months, that was greater in terms of percentage points in the RTX group. These differences were not significant, except with regard to the reduction of ESR levels (greater in the RTX group [P=.023]). The EULAR response rates are shown in Fig. 1.
Changes in the Clinical and Serological Parameters at 6 Months.
| Variable | RTX | 2 TNF | P value |
|---|---|---|---|
| TJC | 4.4±2.4 | 5.8±2.8 | .431 |
| SJC | 3.8±2.7 | 4.4±2.6 | .387 |
| DAS28 | 4.2±2.1 | 4.76±1.9 | .298 |
| ESR (mmHg) | 23.4±17.6 | 33.2±15.6 | .023* |
| CRP (mg/dL) | 11.1±10.7 | 9.8±14.8 | .333 |
| HAQ | 0.82±0.68 | 0.59±0.69 | .960 |
Results expressed as mean±standard deviation and percentages.
CRP, C-reactive protein; DAS28, Disease Activity Score 28 joints; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; RTX, rituximab; SJC, swollen joint count; TJC, tender joint count; TNF, anti-tumor necrosis factor.
A total of 21 patients requested discontinuation of their treatment (11 in the RTX group and 10 from the 2TNF group). In all, 3 patients (27%) in the RTX group did not complete the 6-month evaluation because they had experienced a mild adverse effect, as did another 3 (30%) in the 2TNF group, without evident statistically significant differences (P=.457). There were no severe adverse effects in any group. The rest of the patients were excluded from the study due to the presence of a decline or persistence of symptoms that meant a change in the therapeutic strategy on the part of the rheumatologist in 73% in the RTX group and 70% in the 2TNF group.
DiscussionThis is a prospective clinical practice study, that compares the short-term efficacy of RTX with an anti-TNF in a second BT line after a failure in an anti-TNF in patients with RA.
Overall, the efficacy of RTX was good, with an elevated proportion of patients with a good/moderate EULAR response (64%), comparable to the 59% of 2TNF, even after receiving only a single cycle of RTX, as the evaluation of the clinical efficacy was performed prior to the primary administration of the second cycle corresponding to the 6 months. Moreover, the population who received RTX had a higher activity level according to the DAS28 score, even higher than that of the comparative group (5.45 vs 5.18, respectively). It is important to point out that the majority of these patients discontinued their previous anti-TNF due to a primary inefficacy, something that suggests previous observations.13
Although we found no significant clinical or functional differences, we can observe that RTX shows, as least, the same efficacy as a 2TNF in clinical practice, in a short follow-up time (6 months). At the biological level, we did encounter a higher reduction of the ESR levels in the RTX group, very similar to findings published by Finckh et al.5 This group found greater differences in efficacy in patients treated with RTX in a second BT line than those treated with 2TNF, with a sample size very similar to that of our study, something that was also reported in subsequent explorations.13 These observations were also examined in our population by Gomez-Reino et al.,14 in an observational study at 6, 9 and 12 months in patients with a failure of 1 or more anti-TNF, and those authors found significant differences between the 2 therapeutic strategies. In any case, this last study did find differences in the reduction in DAS28 in those patients who had been previously treated with infliximab and adalimumab. In a later study, the response was evaluated at the radiographic level, and there were no differences between the use of RTX or an 2TNF after the failure of an anti-TNF.15
The limitations of our study are, mainly, the size of the sample (which limits its analysis by subgroups), the higher baseline activity in the RTX group, the design in the clinical setting, the follow-up period and the absence of radiologic evaluations. However, on the other hand, the prospective nature grants our findings greater value than that of other reports published in this same line.
In conclusion, our data demonstrate that, in a second BT line, after the failure of a primary anti-TNF utilized in the clinical setting, RTX can be, at least, as useful as a second anti-TNF.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of InterestThe authors declare they have no conflicts of interest.
The authors wish to thank Ms. Montserrat Sospedra for her invaluable help.
Please cite this article as: Torrente-Segarra V, Acosta Pereira A, Morla R, Ruiz JM, Clavaguera T, Figuls R, et al. Estudio VARIAR: VAloración de la eficacia y seguridad a corto plazo en artritis reumatoide del uso de RItuximab comparado con Antagonistas del factor de necrosis tumoral alfa en segunda línea terapéutica en pacientes con artritis reumatoide Refractarios a un primer antagonista del factor de necrosis tumoral alfa. Reumatol Clin. 2016;12:319–322.