Making the right and timely diagnosis of systemic vasculitis (SV) is often a challenge, even for the most experienced physicians. The pleural condition produced by SV is usually the result of the underlying lung disease. Endothelial damage produced by the involved inflammatory mechanisms increases capillary permeability to protein and fluid towards the pleural cavity. So far, the characteristics of the pleural fluid resulting from the SV have not been properly defined. We will present the case of a male subject who started having a pleural effusion attributed to SV.
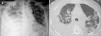
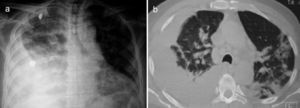
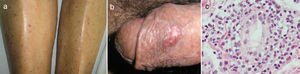
A 67-year-old male subject was hospitalised due to one-week course dyspnoea and fever. Upon admission, the patient was in poor general condition and with a SatO2 of 78% at room temperature. His vital signs were the following: RR 29min, HR 112min, BP 145/85mmHg and a temperature of 37°C. Physical examination showed a right-sided pleural effusion syndrome and very scarce fine crackles in the left hemithorax. The chest X-ray and computed tomography showed bilateral alveolar opacities and verified the presence of a right-sided pleural effusion (Fig. 1). Laboratory results only showed leukocytosis of 21,400cells/μL, out of which 9400cells/μL corresponded to lymphocytes. The patient showed no increased nitrogen compounds, and the general urinalysis showed no signs of sediment or proteinuria. In thoracentesis, a thick yellow fluid was obtained with a pH 7.0; protein 6g/dL; glucose 22mg/dL; LDH 1.066U/L, scarce polymorphonuclear cells without the presence of Gram staining bacteria. The patient was initially treated for pneumonia associated with a complicated pleural effusion and was intubated through the right hemithorax and started receiving IV moxifloxacin 400mg every 24h and IV meropenem 1g every 8h; subsequently, said schedule was replaced by IV vancomycin 500mg every 6h and IV piperacillin 4g/tazobactam 0.5g every 6h. However, during the following days no clinical or radiological improvement was observed, and pleural fluid drainage persisted despite said antimicrobial schedules. The results of the acid-fast bacilli smear, pleural fluid cultures, and blood cultures were negative. After a lengthy hospital stay, the patient showed systemic dermatosis (Fig. 2a), and in a skin biopsy, a lymphocytic vasculitis (Fig. 2c) was documented. This scenario was initially attributed to an antimicrobial adverse effect. Viral hepatitis B and C, HIV, VDRL, pANCA and cANCA profiles were all negative. It was only after a detailed physical exam that some ulcers were found in the penis (Fig. 2b); however, the result of the pathergy test was not conclusive. The case was diagnosed as an incomplete form of Behçet's disease versus ANCA-negative Wegener's granulomatosis; for this reason, the patient was treated with IV methylprednisolone 125mg every 6h, showing a rapid improvement in his general and breathing conditions, which enabled the successful removal of thoracic intubation and hospital discharge soon after having started treatment. Unluckily, the patient died a few weeks later due to a clinical picture of diffuse alveolar haemorrhage.
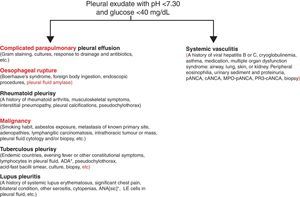
We presented the case of a patient with a complex, active, systemic vasculitic syndrome, with no kidney compromise, with inconclusive lab results, and associated with a pleural effusion not attributed to sequelae, comorbidities, or complications of any medical treatment, which eventually resulted in a fatal outbreak or relapse a few weeks following discharge. From the clinical point of view, it poses a diagnostic challenge given its low prevalence and the unspecified nature of the symptoms, which may overlap with those produced by other diseases, including the different types of SV. In spite of the fact that the presence of pleural effusion may not represent a major criterion of the SV clinical spectrum, it may indeed delay diagnosis and treatment on many occasions, causing disastrous results, mainly in sites where significant similarities with diseases featuring a higher prevalence, such as tuberculosis,1 exist. Pleural effusion has hardly ever been reported2 as a form of presentation of SV; however, the incidence of this complication on Wegener's disease has been described to be in the range of 5%–55% and on Behçet's disease up to 20%.2,3 Nevertheless, as pleural effusion could be attributed to vasculitis itself, other clinical conditions that are often associated with SV, such as thrombosis, infections, and heart or kidney failure, should be excluded first. Up to date, there exists no distinctive cytochemical profile of pleural effusion produced by SV. The presence of exudate acidosis with a low concentration of glucose has suggested the diagnosis of another type of SV and, likewise, the closed pleural biopsy has occasionally contributed to showing pleural vasculitis.4,5 Similarly, and as mentioned hereinbefore, it is imperative to consider other clinical entities showing pleural fluid acidosis and low glucose (Fig. 3) since vasculitic pleural effusion treatment and prognosis correspond to those of the underlying disease. Therefore, we suggest that the physician pay attention to the presence of those clinical findings suggestive of SV in each and every patient showing pleural fluid acidosis of unknown origin.
Study proposal of the patient with pleural fluid acidosis and low glucose concentration. Diagnostic priorities will always be the entities mentioned on the left. Vasculitic pleural effusion will always be a diagnosis of exclusion of the said diseases. ANA, antinuclear antibodies; ADA, adenosine deaminase.
Please cite this article as: Flores-Franco RA, Ramos-Martínez E. Derrame pleural vasculítico. Reumatol Clin. 2015;11:186–187.