Axial spondyloarthritis (axSpA) is characterized by new bone formation. The complex systems underlying this process involve Wnt-signaling pathway. It has been observed that serum levels of dickkopf-1 (DKK-1), an important inhibitor of Wnt-signaling, are decreased in patients with axSpA. However, these data are from studies including only patients with long-standing disease.
The aim of this study is to investigate if symptom duration influences on serum DKK-1 levels in patients with axSpA.
Material and methodsA cross-sectional study including consecutive patients with axSpA (ASAS criteria) naïve for anti-TNF therapy. Collected data included demographic and disease characteristics, time since first symptom onset, assessment of disease activity and function, and determination of DKK-1 serum levels. Patients were classified as early axSpA (symptom duration ≤5 years) and established axSpA (>5 years). Linear regression models were employed to investigate the variables related to DKK-1 serum levels.
ResultsIn total, 90 patients were included. Sixty-eight patients had early axSpA and 22 had established disease. Serum levels of DKK-1 were significantly higher in patients with early axSpA compared with established axSpA (22.1±12.6 vs 16.4±10.7pM; p=0.04). Among all tested variables, only symptom duration was significantly and inversely correlated with DKK-1 serum levels (beta: −0.041; p=0.01).
ConclusionSerum DKK-1 levels in axSpA depend on disease duration. As disease duration increases, DKK-1 serum levels decrease. Based on this, an intensive treatment at early stages of the disease could have a better outcome on inhibiting/slowing radiographic progression in patients with axSpA.
Espondiloartritis axial (EsPax) se caracteriza por nueva formación ósea. El complejo sistema que subyace este proceso incluye la vía de señal Wnt. Se ha demostrado que niveles séricos de dickkopf-1 (DKK-1), un importante inhibidor de la vía de señal Wnt, está disminuidos en pacientes con EsPax. Sin embargo, estos datos proceden exclusivamente de pacientes con EsPax de larga duración.
El objetivo de este estudio es investigar si la duración de la enfermedad influye en niveles séricos de DKK-1 en pacientes con EsPax.
Material y métodosEstudio transversal en pacientes con EsPax sin terapia anti-TNF. Se recogieron datos demográficos y de la enfermedad, y se determinaron niveles de DKK-1 séricos en la misma visita. Los pacientes fueron clasificados en base a la duración de síntomas en EsPax precoz (≤5 años) y establecida (>5 años). Se emplearon modelos de regresión lineal para investigar las variables asociadas con los niveles de DKK-1.
ResultadosSe incluyeron 90 pacientes, 68 con EsPax precoz y 22 con EsPax establecida. Los niveles de DKK-1 fueron superiores en EsPax precoz comparado con EsPax establecida (22.1±12.6 vs 16.4±10.7pM; p=0.04). De todas las variables, sólo la duración de síntomas se asoció significativamente con DKK-1 (beta: −0.041; p=0.01).
ConclusionesLos niveles séricos de DKK-1 en EsPax, dependen de la duración de la enfermedad. A medida que la duración de la enfermedad aumenta, niveles séricos de DKK-1 disminuye. Por lo tanto, el tratamiento intensivo en estadios tempranos de la enfermedad podría tener un mejor resultado en inhibir/disminuir la progresión radiológica en pacientes con EsPax.
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease that mainly affects the spine and sacroiliac joints. Structural damage in axSpA is characterized by new bone formation leading to syndesmophytes, bone bridges and complete ankylosis. The complex systems that underlie new bone formation in patients with axSpA involve Wingless protein (Wnt) signaling and growth factors such as the bone morphogenetic proteins (BMPs).
An important inhibitor of the Wnt signaling is dickkopf-related protein 1 (DKK-1), which seems to be enhanced by tumor necrosis factor alpha (TNF). Serum DKK-1 levels are decreased in patients with axSpA compared with healthy controls1 and other rheumatic inflammatory diseases such as rheumatoid arthritis.2 Additionally, it has been shown that DKK-1 serum levels are related to radiographic progression in patients with axSpA.3 Specifically, high levels of functional DKK-1 seems to be protective of the development of syndesmophytes,4 and DKK-1 blockade leads to the fusion of sacroiliac joints in an animal model of arthritis.5
Furthermore, the mechanisms and characteristics influencing serum DKK-1 levels in patients with axSpA are unclear. In principle, the most accepted hypothesis is that TNF induces DKK-1,6 which down-regulates bone formation through inhibition of Wnt signaling. However, the change produced in DKK-1 serum levels after treatment with TNF blockers is not yet conclusive since different results (decrease, increase and even no change on DKK-1) have been reported in this sense.7–9 In addition, TNF blockers have shown clinical efficacy in patients with axSpA,10 but inhibition or slowing radiographic progression with this therapy has not been demonstrated.11–13 Based on this, it may be hypothesized that inflammation and radiographic progression are uncoupled processes in axSpA.
Furthermore, all these data come from studies including patients with long-standing and established disease.11–13 The influence of disease duration on DKK-1 levels is unknown since no study has previously assessed whether or not patients at early stages of the disease have the same decreased levels of DKK-1 than patients with longstanding and established disease. Based on this, the aim of this study is to investigate if disease duration influences on serum levels of DKK-1 in patients with axSpA.
Materials and methodsStudy design, population and data collectionA cross-sectional study in which consecutive patients with axSpA attending the outpatient clinic of a tertiary hospital between January 2011 and June 2014 were included. Patients should fulfill Assessment of SpondyloArthritis International Society (ASAS) classification criteria for axSpA and be naïve for treatment with TNF blockers or other biological therapy.
The following data were collected: demographic characteristics including age and gender; disease characteristics specifying symptoms duration, human leukocyte antigen B27 (HLA-B27) and presence or previous history of SpA features including psoriasis, inflammatory bowel disease, uveitis or peripheral arthritis; and concomitant treatment. Disease Activity was measured by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the Ankylosing Spondylitis Disease Activity Score (ASDAS), patient's visual analog score of disease activity (Patient's VAS) and C-Reactive Protein (CRP). Enthesitis was assessed using the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) and function was evaluated through the Bath Ankylosing Spondylitis Functional Index (BASFI). A physical examination was also performed to assess mobility, measured by the Bath Ankylosing Spondylitis Mobility Index (BASMI). Blood samples to determine Dkk-1 serum levels by enzyme immunoassay were collected too. In addition, based on symptoms duration patients were classified in two groups: early axSpA (less than or equal to 5 years) and established axSpA (more than 5 years). This study was approved by the local ethical committee, and all patients signed a written informed consent before being included.
Quantification of dickkopf-1 (Dkk-1) levelsSerum samples were obtained through venous punctures and stored at −80°C until assays were performed. Commercially available kit was used (Biomedica Medizinprodukte GmbH & Co KG, A-1210 Wien, Divischgasse 4). Serum samples were diluted 2:7 and were processed by an enzyme immunoassay. DKK-1 levels were estimated by means of colourimetric measurements at 450nm and by interpolation from a standard curve (0–160pM) on an ELISA plate reader, obtaining DKK-1 serum concentrations in pM. The intra- and inter-assay coefficients of variation were <3%.
Statistical analysisFirst, a descriptive analysis was performed. For this purpose, collected variables between patients with early and established axSpA were compared using Student t-test for continuous variables and chi-square test for categorical variables. The results are presented as mean and standard deviation (SD) for continuous variables and as percentage and relative frequencies for categorical variables. Second, separate linear regression models adjusted for age and gender were employed for identifying the variables related to serum levels of DKK-1. For all the analyses, SPSS software version 21.0 was employed and p-values less than 0.05 were considered statistically significant.
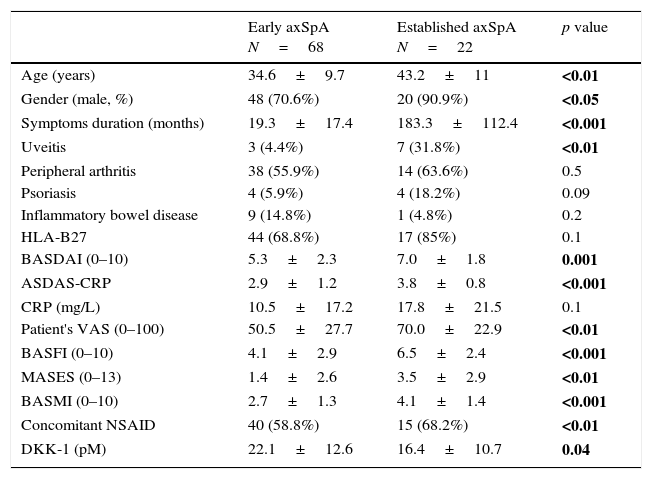
ResultsNinety patients with axSpA were included in this study. Sixty-eight patients were classified as early axSpA and 22 as established disease. Results for demographic, disease characteristics and biomarkers in each group of patients are shown in Table 1. Compared to patients with established disease, patients with early axSpA were younger, had a higher proportion of females, lower degree of disease activity and less enthesis involvement. As expected, they also had higher mobility range aside from a better function.
Demographic, disease characteristics and biomarkers based on symptoms duration.
| Early axSpA N=68 | Established axSpA N=22 | p value | |
|---|---|---|---|
| Age (years) | 34.6±9.7 | 43.2±11 | <0.01 |
| Gender (male, %) | 48 (70.6%) | 20 (90.9%) | <0.05 |
| Symptoms duration (months) | 19.3±17.4 | 183.3±112.4 | <0.001 |
| Uveitis | 3 (4.4%) | 7 (31.8%) | <0.01 |
| Peripheral arthritis | 38 (55.9%) | 14 (63.6%) | 0.5 |
| Psoriasis | 4 (5.9%) | 4 (18.2%) | 0.09 |
| Inflammatory bowel disease | 9 (14.8%) | 1 (4.8%) | 0.2 |
| HLA-B27 | 44 (68.8%) | 17 (85%) | 0.1 |
| BASDAI (0–10) | 5.3±2.3 | 7.0±1.8 | 0.001 |
| ASDAS-CRP | 2.9±1.2 | 3.8±0.8 | <0.001 |
| CRP (mg/L) | 10.5±17.2 | 17.8±21.5 | 0.1 |
| Patient's VAS (0–100) | 50.5±27.7 | 70.0±22.9 | <0.01 |
| BASFI (0–10) | 4.1±2.9 | 6.5±2.4 | <0.001 |
| MASES (0–13) | 1.4±2.6 | 3.5±2.9 | <0.01 |
| BASMI (0–10) | 2.7±1.3 | 4.1±1.4 | <0.001 |
| Concomitant NSAID | 40 (58.8%) | 15 (68.2%) | <0.01 |
| DKK-1 (pM) | 22.1±12.6 | 16.4±10.7 | 0.04 |
BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; ASDAS: Ankylosing Spondylitis Disease Activity Score; CRP: C Reactive Protein; Patient's VAS: patient's global visual analog scale; BASFI: Bath Ankylosing Spondylitis Functional Index; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; BASMI: Bath Ankylosing Spondylitis Metrology Index; NSAID: Nonsteroidal Anti-Inflammatory Drugs; DKK-1: Dickkopf-1. Results are shown as mean±standard deviation or absolute number with percentages. p value in bold: with statistical significance.
Importantly, DKK-1 serum levels were found to be statistically significant higher in patients with early axSpA compared with patients with established disease (22.1±12.6 vs 16.4±10.7pM; p=0.04, respectively).
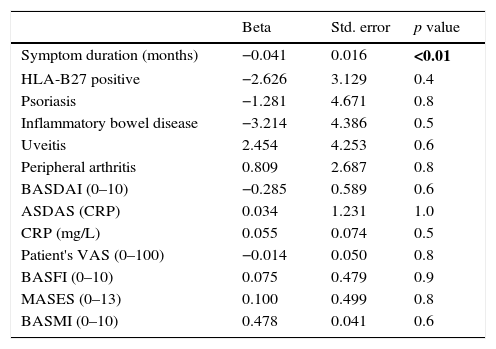
Furthermore, Table 2 shows the results for the separate linear regression models evaluating the association between DKK-1 serum levels and symptom duration, disease activity, function and mobility. Among all the tested variables, only symptom duration was statistically significant related to DKK-1 serum levels (standard β: −0.041; p=0.01). This relationship was inverse: as disease duration increased, DKK-1 serum levels decreased.
Separate linear regression models for the association between DKK-1 and disease characteristics.
| Beta | Std. error | p value | |
|---|---|---|---|
| Symptom duration (months) | −0.041 | 0.016 | <0.01 |
| HLA-B27 positive | −2.626 | 3.129 | 0.4 |
| Psoriasis | −1.281 | 4.671 | 0.8 |
| Inflammatory bowel disease | −3.214 | 4.386 | 0.5 |
| Uveitis | 2.454 | 4.253 | 0.6 |
| Peripheral arthritis | 0.809 | 2.687 | 0.8 |
| BASDAI (0–10) | −0.285 | 0.589 | 0.6 |
| ASDAS (CRP) | 0.034 | 1.231 | 1.0 |
| CRP (mg/L) | 0.055 | 0.074 | 0.5 |
| Patient's VAS (0–100) | −0.014 | 0.050 | 0.8 |
| BASFI (0–10) | 0.075 | 0.479 | 0.9 |
| MASES (0–13) | 0.100 | 0.499 | 0.8 |
| BASMI (0–10) | 0.478 | 0.041 | 0.6 |
BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; ASDAS: Ankylosing Spondylitis Disease Activity Score; CRP: C Reactive Protein; Patient's VAS: patient's global visual analog scale of disease activity; BASFI: Bath Ankylosing Spondylitis Functional Index; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; BASMI: Bath Ankylosing Spondylitis Metrology Index; Beta: unstandardized beta coefficient; Std. error: standard error. All models are adjusted for age and gender. p value in bold: with statistical significance.
This study shows that DKK-1 serum levels in patients with axSpA depend on symptom duration. Independently of the age and gender, patients with early axSpA have higher serum DKK-1 levels compared with patients with established disease.
These findings are consistent with published data, in which low serum levels of DKK-1 in patients with established ankylosing spondylitis have been observed.1,14,15 On the other hand, opposite to a previous study, an association between disease activity and DKK-1 levels was not found.15 In our study, most patients had active or very active disease activity while in the study of Korskos et al. the degree of disease activity was more heterogeneous. This may explain the difference on the results between the studies.
The difference on DKK-1 serum levels found in our study between patients with early and established axSpA may be relevant to understand why despite suppressing inflammation, TNF blockers have not shown to inhibit the development of syndesmophytes in patients with axSpA. On one hand, disease activity has been longitudinally related to radiographic progression in patients with axSpA.16 On the other hand, TNF blockers decrease disease activity but have not shown to inhibit new bone formation in patients with axSpA.11–13
In addition, it has been shown that low levels of serum DKK-1 are necessary to develop syndesmophytes.4 What is unclear is the relationship between TNF and DKK-1. TNF stimulates DKK-1 but previous studies have found that inhibition of TNF by TNF-blockers does not necessarily lead to the decrease of serum DKK-1.7,9 This highlights the fact that there may be more important factors different than TNF regulating DKK-1 production. As shown in the results of our study, in patients with established axSpA DKK-1 serum levels are already decreased.17 This may explain why despite suppressing disease activity, the administration of a more intensive therapy such as TNF-blockers in a late stage of the disease is not able to avoid the development of syndesmophytes. Nevertheless, this situation may be different in patients at an early stage of the disease, in which DKK-1 serum levels are not decreased yet. Based on this, initiation of TNF-blockers at early stages of the disease in patients with axSpA could have a different (better) outcome on inhibiting or slowing radiographic progression. So, these results support the hypothesis of a window of opportunity to treat patients with axSpA if the avoidance of irreversible radiographic damage is desired.
The following process has been postulated to explain how inflammation could lead to new bone formation in axSpA18: First, the initial development of inflammation causes erosive cartilage and bone destruction. Later, these lesions are filled in by fibrous tissue. And finally, this tissue is ossified leading to syndesmophytes. This process is mediated by TNF-alfa and Dkk-1. TNF-blockers target the sites of active inflammation but have no direct effects on osteoproliferation.18 In other words, it could be postulated that TNF-blockers block the transformation from inflammation to erosion; however, they don’t block the transformation from erosion to osteoproliferation, where non-steroidal anti-inflammatory drugs might have a role.19 This may explain why initiation of TNF-blockers at early stages of the disease could have a better outcome on the development of radiographic progression.
Furthermore, several limitations of this study should be taken into account when interpreting its results. First, this is a cross-sectional study, which does not allow investigating if DKK-1 serum levels truly decrease overtime. Second, no control group was included so it is not possible to know whether patients with early axSpA have similar or lower serum levels of DKK-1 than healthy population. Third, the group of patients with established disease was substantially smaller than the group with early disease and a bigger sample size in this group could have an impact. Finally, no radiographs were available to assess radiographic damage or progression in these patients.
In summary, DKK-1 serum levels depend on symptom duration in patients with axSpA. Patients with early disease have higher serum DKK-1 levels compared with patients with established axSpA. Based on the results of the current study, it can be concluded that serum DKK-1 levels are not decreased from the beginning of the disease in patients with axSpA but they decrease as disease duration increases. This could underlie a window of opportunity to avoid development of new bone formation through TNF-blockers in patients with axSpA. Nevertheless, further data from longitudinal studies are necessary to confirm this.
Ethical responsibilitiesProtection of people and animalsThe authors state that the procedures conformed to the ethical standards of human experimentation committee responsible and according to the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of the workplace on the publication of patient data.
Right to privacy and informed consentThe authors declare that this article does not appear patient data.
FundingThis work was supported by an Initiated Investigator Research (IIR) grant from Pfizer, the Institute Carlos III (PI10/01963) and the Spanish Society of Rheumatology (call 2010).
Conflicts of interestAll authors declare no conflicts of interest in this manuscript.
We are grateful to all the patients that participated in this study for their collaboration. In addition, we would like to thank Cristian Leyva, Rufino Mondéjar, Virginia Moreira and Consuelo Leon, who participated in the determination and collection of clinical and laboratory variables. We also thank Ismael Gómez for medical advisory support.