Systemic sclerosis (SSc) is characterized by progressive fibrosis of the skin and internal organs, microvascular damage and cellular and humoral immunity abnormalities. Microvascular damage can be easily detected through nailfold videocapillaroscopy (NVC).
Materials and methodsA retrospective study of patients with SSc and a NVC performed within the first 6 months after diagnosis was conducted. Visceral involvement in the first 3 years of the disease and NVC findings were collected. The severity of microvascular damage was classified into four categories, according to the worsening of the NVC patterns. The severity of organ involvement was assessed by the disease severity scale of Medsger for each organ and as a global measure of disease severity, the simple summation was used.
ResultsA total of 86 patients with SSc were included. A moderate correlation was found between the severity of microvascular damage and the global measure of disease severity (r=0.55, p<0.001), the severity of peripheral vascular involvement (r=0.43, p<0.001) and the severity of skin involvement (r=0.34, p=0.001).
The presence of a late scleroderma pattern in NVC were predictive in univariate analysis of digital ulcers (OR 6.03, 95% CI 1.52–23.86, p=0.01), muscular involvement (OR 13.09, 95% CI 1.09–156.78, p=0.04), calcinosis (OR 27.22, 95% CI 5.56–133.33, p<0.001) and worse global disease severity score (OR 1.67, 95% CI 1.17–2.38, p=0.005). Multivariate analysis adjusted for disease duration and gender confirmed late pattern as an independent predictor of calcinosis (OR 42.89, 95% CI 5.53–332.85, p<0.001).
Discussion and conclusionIn this study, the worsening of NVC pattern in SSc was associated with the overall disease severity, the severity of peripheral vascular involvement and extension of skin involvement. This study highlights the importance of NVC as a prognostic tool and a possible predictor of systemic visceral involvement.
La esclerosis sistémica (ES) se caracteriza por fibrosis progresiva de la piel y órganos internos, daño microvascular y anomalías en la inmunidad celular y humoral. El daño microvascular puede detectarse fácilmente mediante la capilaroscopia periungueal (CPU).
Materiales y métodosUn estudio retrospectivo en pacientes con ES que realizaron una CPU en los primeros 6 meses después del diagnóstico. Se recopilaron los hallazgos de la CPU y el compromiso visceral en los primeros 3 años de la enfermedad. La gravedad del daño microvascular se clasificó en 4 categorías, según el empeoramiento de los patrones de la CPU. La gravedad del compromiso orgánico se evaluó mediante la escala de gravedad de la enfermedad de Medsger para cada órgano, y como medida global de la gravedad de la enfermedad, se utilizó la suma simple.
ResultadosSe incluyeron un total de 86 pacientes con ES. Se encontró una correlación moderada entre la gravedad del daño microvascular y la medida global de la gravedad de la enfermedad (r=0,55; p<0,001), la gravedad del compromiso vascular periférico (r=0,43; p<0,001) y la gravedad del compromiso cutáneo (r=0,34; p=0,001).
La presencia de un patrón esclerodérmico tardío en la CPU fue predictiva en el análisis univariado de úlceras digitales (OR: 6,03; IC 95%: 1,52-23,86; p=0,01), compromiso muscular (OR: 13,09; IC 95%: 1,09-156,78; p=0,04), calcinosis (OR: 27,22; IC 95%: 5,56-133,33; p<0,001) y peor puntuación global de la gravedad de la enfermedad (OR: 1,67; IC 95%: 1,17-2,38; p=0,005). El análisis multivariado, ajustado por la duración de la enfermedad y el género, confirmó el patrón tardío como un predictor independiente de la calcinosis (OR: 42,89; IC 95%: 5,53-332,85; p<0,001).
Discusión y conclusiónEn este estudio, el empeoramiento del patrón de la CPU en la ES se asoció con la gravedad global de la enfermedad, la gravedad del compromiso vascular periférico y la extensión del compromiso cutáneo. Este estudio destaca la importancia de la CPU como una herramienta pronóstica y un posible predictor del compromiso visceral sistémico.
Systemic sclerosis (SSc) is a rare autoimmune disease, with a prevalence in Europe ranging from 7.2 to 33.9 cases per 100,000 individuals.1 This disease is characterized by progressive fibrosis of the skin and internal organs, microvascular damage and cellular and humoral immunity abnormalities. It primarily involves the microcirculation and Raynaud's phenomenon (RP) is typically the first and most prevalent manifestation of the disease, occurring in more than 90% of patients.2
Microvascular damage can be easily detected through nailfold videocapillaroscopy (NVC), which is a simple, safe and non-invasive technique that allows a detailed assessment of nailfold capillaries.3 NVC shows a variety of morphological changes, including microhemorrhages, enlarged and giant capillaries, impaired capillary architecture, loss of capillaries with or without avascular areas and the presence of abnormal shapes.4 Some authors have disclosed specific capillaroscopic patterns in SSc patients, known as “scleroderma patterns”. These patterns not only aid in the diagnostic process, but also appear to correlate with consecutive stages of capillary damage.4,5 The three NVC scleroderma patterns (early, active and late) seem to reflect the evolution of SSc microangiopathy and may be useful in assessing microvascular damage. Therefore, NVC can be a diagnostic and a follow-up parameter to track microvascular changes over time.
However, there has been limited research and understanding regarding the impact of the severity of microvascular damage in predicting overall disease severity. With the aim of assessing NVC as a marker of overall disease severity, this study was conducted to evaluate microvascular damage using NVC among patients with SSc at disease onset and to assess associations between these changes and systemic involvement over a 3-year follow-up period.
Material and methodsStudy designAn observational retrospective cohort study was conducted with 3 years of follow-up.
ParticipantsPatients aged 18 years or older, classified as having SSc according to the 2013 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria,6 followed at a single tertiary rheumatology centre were included. Those with psychiatric or cognitive disorders that could potentially interfere with data collection, as well as patients who were physically or psychologically unable to communicate, were excluded.
The Guideline for Good Clinical Practice of the International Conference on Harmonization and the ethical principles of the Declaration of Helsinki were followed. Each patient signed informed consent and all data were anonymised in accordance with both the Portuguese Data Protection Law and the General Data Protection Regulation.
Data collection and measuresNVC assessmentNVC was performed by the same experienced rheumatologist with the same equipment in the first 6 months after the SSc diagnosis. The nailfolds of the second to fifth fingers were examined bilaterally in each patient. Detailed information regarding the number of capillaries, presence of giant capillaries, haemorrhages, avascular areas and abnormal shapes were collected. NVC assessments were conducted in accordance with the standardized capillaroscopy evaluation chart established by the EULAR Study Group on Microcirculation in Rheumatic Diseases.7 Initially, capillaroscopic parameters were classified according to the CAP Fast Track Algorithm in “scleroderma pattern” or “non-scleroderma pattern” (including “normal pattern” and “non-specific abnormalities”).8 Subsequently, the NVC with a scleroderma pattern was further stratified into early, active or late pattern. The severity of microvascular damage was graded into four distinct categories, based on the worsening of the NVC patterns (0=non-scleroderma pattern, 1=early pattern, 2=active pattern and 3=late pattern).
Clinical and laboratory measurementsSociodemographic, clinical evaluations and laboratory findings at disease onset were obtained by consulting medical records and Rheumatic Diseases Portuguese Registry (Reuma.pt). The following clinical data were collected for all patients: age, gender, smoking and drinking habits and comorbidities. Regarding SSc-related details, data on disease duration, age at diagnosis and laboratory data, including tests for specific autoantibodies (such as anticentromere and antitopoisomerase-I antibodies, among others) were obtained.
To assess the extent of systemic involvement of SSc patients, data on various visceral manifestations within the first 3 years after the diagnosis was collected. Visceral involvement was defined as follows:
- -
Pulmonary involvement: evidence of interstitial lung disease (ILD) on high-resolution computed tomography (CT) and/or restrictive lung disease on pulmonary function tests.
- -
Cardiac involvement: evidence of severe arrhythmias, cardiac conduction blocks, pericardial effusion (>10mm and diffuse), systolic (ejection fraction <50%), or diastolic dysfunction (according to the ASE criteria9).
- -
Pulmonary arterial hypertension (PAH): evidence of a mean pulmonary arterial pressure ≥25mmHg measured by right heart catheterization with a pulmonary artery wedge pressure ≤15mmHg.
- -
Gastrointestinal involvement: presence of dysphagia and esophageal dysmotility on manometry, symptoms of gastroesophageal reflux, constipation, pseudo-obstruction, or diarrhoea.
- -
Renal involvement: history of scleroderma renal crisis (SRC) and/or progressive renal failure.
- -
Muscle involvement: evidence of myositis in magnetic resonance imaging, electromyography or muscle biopsy.
- -
Joint involvement: presence of inflammatory polyarthralgia and/or arthritis.
- -
Skin involvement: presence of skin thickness assessed by the modified Rodnan skin score (mRSS).10
- -
Peripheral vascular involvement: occurrence of digital ulcers (DU) or/and RP.
The severity of visceral involvement was assessed using the Medsger's disease severity scale (DSS).11 The Medsger DSS comprises separate clinician ratings for nine domains: (1) general domain assessing weight and anaemia; (2) peripheral vascular domain assessing RP and digital ischaemia; (3) joint/tendon domain assessing flexed finger to palm distance; (4) muscle domain assessing weakness; (5) gastrointestinal (GI) domain assessing esophageal and small intestinal involvement; (6) pulmonary domain assessing the degree of fibrosis and pulmonary hypertension; (7) cardiac domain assessing arrhythmias and cardiac function; (8) renal domain assessing renal function, proteinuria and the need for dialysis and (9) skin domain assessing skin thickness. Each organ system is graded on a scale from 0 (no involvement) to 4 (end-stage involvement). If the organ grade was 2 or higher, it was classified as severe involvement. To provide a global measure of disease severity, the simple summation of the Medsger DSS for each domain was used.
Statistical analysisDescriptive statistics for normally distributed continuous variables were presented using the mean and standard deviation. Categorical variables were presented by absolute and relative frequencies. To compare groups across the four stages of microvascular damage, the Chi-square test was employed for categorial variables, while the T-test or ANOVA were utilized for normally distributed continuous variables, and the Mann–Whitney U test was applied for continuous data that did not follow a normal distribution.
Pearson correlations were performed to assess the association between the severity of microvascular damage and the severity of visceral involvement for each domain of the Medsger DSS. Correlations were categorized as weak (r<0.30), moderate (r=0.30–0.69) or strong (r>0.70). Univariate and multivariate logistic regression analysis were also conducted and odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs) were calculated.
Data analysis was performed using IBM SPSS for Windows (version 26, IBM Corporation Software Group, New York, NY, USA). Statistical significance was defined as a p-value <0.05.
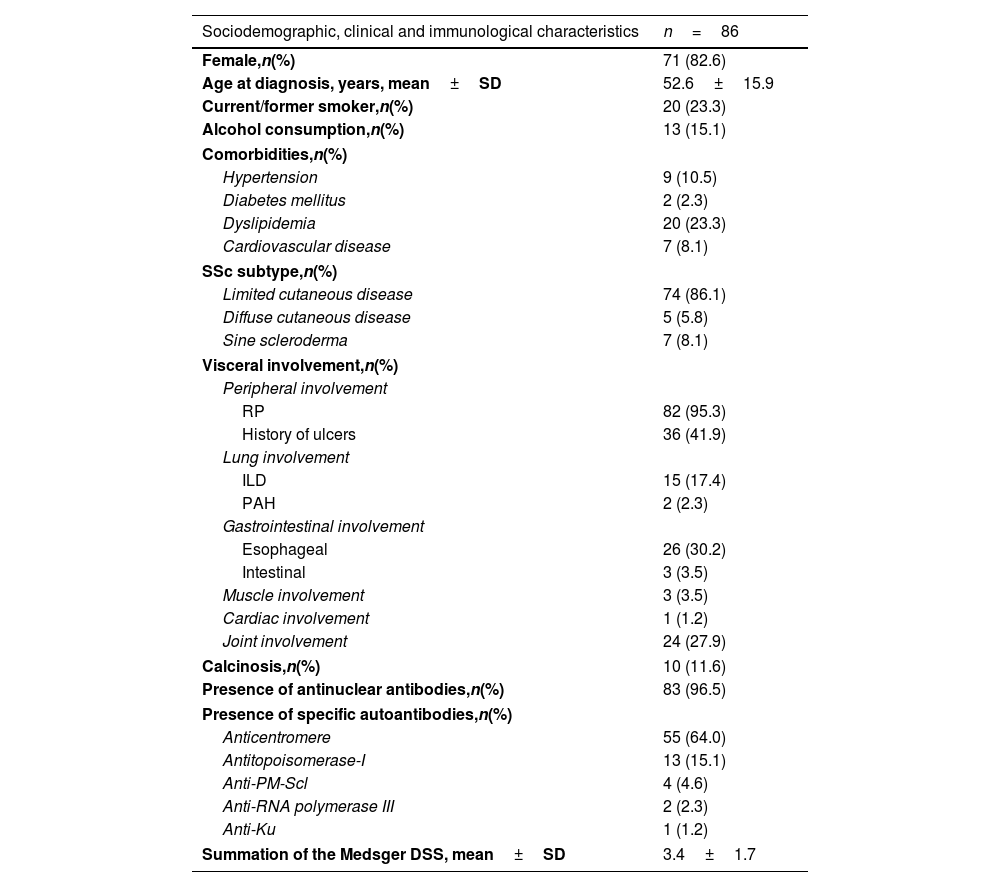
ResultsDemographic and clinical characteristics of SSc patientsA total of 86 patients with SSc were included, comprising 71 females (82.6%) with a mean age of onset of 52.6±15.9 years. Among these patients, 74 (86.1%) had a limited cutaneous disease, 5 (5.8%) had a diffuse cutaneous disease and 7 (8.1%) presented with SSc sine scleroderma. Table 1 provides a comprehensive overview of sociodemographic data, comorbidities, and the clinical and immunological characteristics of SSc patients.
Sociodemographic data, clinical and immunological characteristics at baseline of patients with systemic sclerosis.
| Sociodemographic, clinical and immunological characteristics | n=86 |
|---|---|
| Female,n(%) | 71 (82.6) |
| Age at diagnosis, years, mean±SD | 52.6±15.9 |
| Current/former smoker,n(%) | 20 (23.3) |
| Alcohol consumption,n(%) | 13 (15.1) |
| Comorbidities,n(%) | |
| Hypertension | 9 (10.5) |
| Diabetes mellitus | 2 (2.3) |
| Dyslipidemia | 20 (23.3) |
| Cardiovascular disease | 7 (8.1) |
| SSc subtype,n(%) | |
| Limited cutaneous disease | 74 (86.1) |
| Diffuse cutaneous disease | 5 (5.8) |
| Sine scleroderma | 7 (8.1) |
| Visceral involvement,n(%) | |
| Peripheral involvement | |
| RP | 82 (95.3) |
| History of ulcers | 36 (41.9) |
| Lung involvement | |
| ILD | 15 (17.4) |
| PAH | 2 (2.3) |
| Gastrointestinal involvement | |
| Esophageal | 26 (30.2) |
| Intestinal | 3 (3.5) |
| Muscle involvement | 3 (3.5) |
| Cardiac involvement | 1 (1.2) |
| Joint involvement | 24 (27.9) |
| Calcinosis,n(%) | 10 (11.6) |
| Presence of antinuclear antibodies,n(%) | 83 (96.5) |
| Presence of specific autoantibodies,n(%) | |
| Anticentromere | 55 (64.0) |
| Antitopoisomerase-I | 13 (15.1) |
| Anti-PM-Scl | 4 (4.6) |
| Anti-RNA polymerase III | 2 (2.3) |
| Anti-Ku | 1 (1.2) |
| Summation of the Medsger DSS, mean±SD | 3.4±1.7 |
DSS: disease severity score; ILD: interstitial lung disease; PAH: pulmonary arterial hypertension; RP: Raynaud phenomenon; SD: standard deviation; SSc: systemic sclerosis.
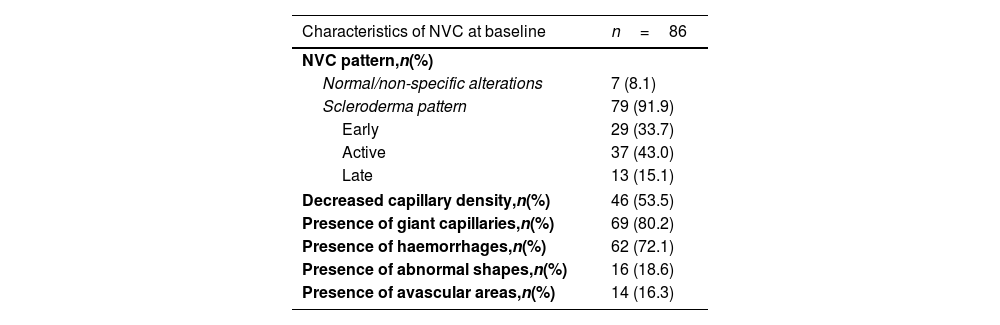
At baseline, NVC revealed a scleroderma pattern in 79 (91.9%) patients. Among these, 29 (33.7%) exhibited an early pattern, 37 (43.0%) had an active pattern and 13 (15.1%) had a late pattern. The remaining 7 patients who did not meet the capillaroscopic criterion had SSc-specific antibodies and presented with suggestive clinical manifestations. Further details related to the NVC characteristics can be found in Table 2.
Nailfold videocapillaroscopy (NVC) characteristics at baseline in patients with systemic sclerosis (SSc).
| Characteristics of NVC at baseline | n=86 |
|---|---|
| NVC pattern,n(%) | |
| Normal/non-specific alterations | 7 (8.1) |
| Scleroderma pattern | 79 (91.9) |
| Early | 29 (33.7) |
| Active | 37 (43.0) |
| Late | 13 (15.1) |
| Decreased capillary density,n(%) | 46 (53.5) |
| Presence of giant capillaries,n(%) | 69 (80.2) |
| Presence of haemorrhages,n(%) | 62 (72.1) |
| Presence of abnormal shapes,n(%) | 16 (18.6) |
| Presence of avascular areas,n(%) | 14 (16.3) |
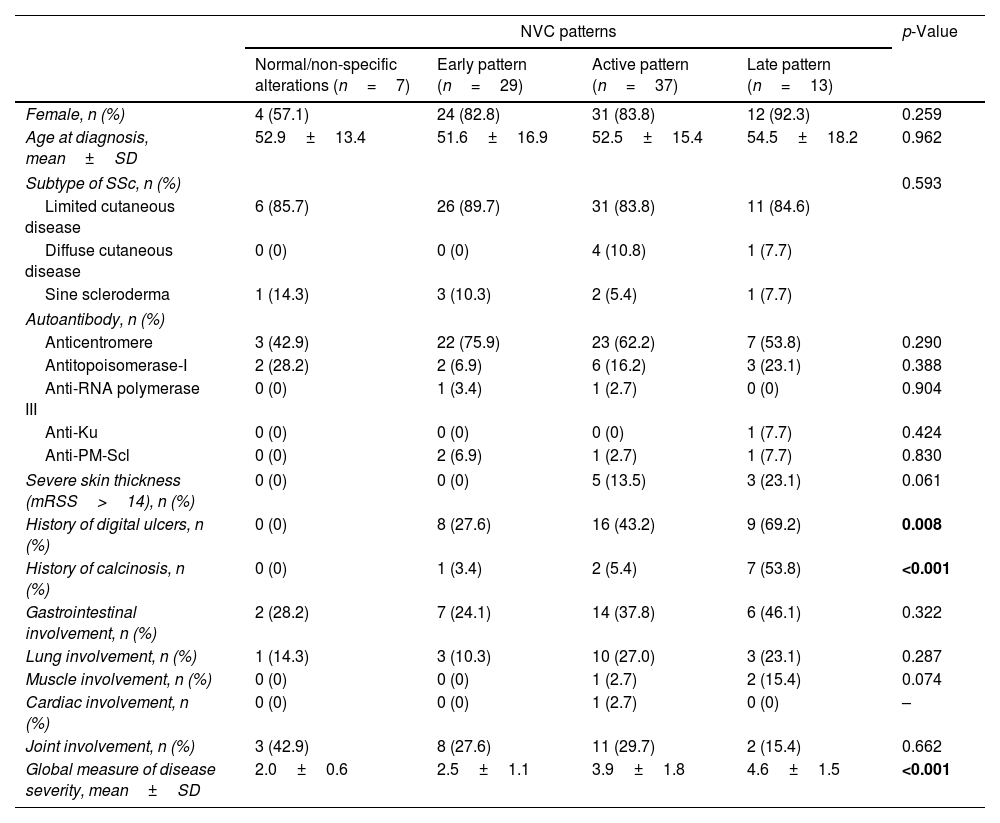
When comparing the four stages of microvascular damage, we found significant differences among these groups concerning the incidence of digital ulcers (p=0.008), calcinosis (p<0.001) and global disease severity score (p<0.001), all of which were higher in patients with a late scleroderma pattern. However, no statistically significant differences were found between groups concerning gender, age at diagnosis, subtype of SSc, autoantibody profile or other visceral manifestations. More detailed information was described in Table 3.
Relationship between severity of microvascular damage, global measure of disease severity and severity of different visceral involvement.
| NVC patterns | p-Value | ||||
|---|---|---|---|---|---|
| Normal/non-specific alterations (n=7) | Early pattern (n=29) | Active pattern (n=37) | Late pattern (n=13) | ||
| Female, n (%) | 4 (57.1) | 24 (82.8) | 31 (83.8) | 12 (92.3) | 0.259 |
| Age at diagnosis, mean±SD | 52.9±13.4 | 51.6±16.9 | 52.5±15.4 | 54.5±18.2 | 0.962 |
| Subtype of SSc, n (%) | 0.593 | ||||
| Limited cutaneous disease | 6 (85.7) | 26 (89.7) | 31 (83.8) | 11 (84.6) | |
| Diffuse cutaneous disease | 0 (0) | 0 (0) | 4 (10.8) | 1 (7.7) | |
| Sine scleroderma | 1 (14.3) | 3 (10.3) | 2 (5.4) | 1 (7.7) | |
| Autoantibody, n (%) | |||||
| Anticentromere | 3 (42.9) | 22 (75.9) | 23 (62.2) | 7 (53.8) | 0.290 |
| Antitopoisomerase-I | 2 (28.2) | 2 (6.9) | 6 (16.2) | 3 (23.1) | 0.388 |
| Anti-RNA polymerase III | 0 (0) | 1 (3.4) | 1 (2.7) | 0 (0) | 0.904 |
| Anti-Ku | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 0.424 |
| Anti-PM-Scl | 0 (0) | 2 (6.9) | 1 (2.7) | 1 (7.7) | 0.830 |
| Severe skin thickness (mRSS>14), n (%) | 0 (0) | 0 (0) | 5 (13.5) | 3 (23.1) | 0.061 |
| History of digital ulcers, n (%) | 0 (0) | 8 (27.6) | 16 (43.2) | 9 (69.2) | 0.008 |
| History of calcinosis, n (%) | 0 (0) | 1 (3.4) | 2 (5.4) | 7 (53.8) | <0.001 |
| Gastrointestinal involvement, n (%) | 2 (28.2) | 7 (24.1) | 14 (37.8) | 6 (46.1) | 0.322 |
| Lung involvement, n (%) | 1 (14.3) | 3 (10.3) | 10 (27.0) | 3 (23.1) | 0.287 |
| Muscle involvement, n (%) | 0 (0) | 0 (0) | 1 (2.7) | 2 (15.4) | 0.074 |
| Cardiac involvement, n (%) | 0 (0) | 0 (0) | 1 (2.7) | 0 (0) | – |
| Joint involvement, n (%) | 3 (42.9) | 8 (27.6) | 11 (29.7) | 2 (15.4) | 0.662 |
| Global measure of disease severity, mean±SD | 2.0±0.6 | 2.5±1.1 | 3.9±1.8 | 4.6±1.5 | <0.001 |
A moderate correlation was found between the severity of microvascular damage and the global measure of disease severity (r=0.55, p<0.001). Also, a moderate correlation was found between the severity of microvascular damage and the severity of peripheral vascular involvement (r=0.43, p<0.001) and patients with a late pattern had a markedly increased risk of digital ulcers (OR 4.6, 95% CI 1.3–16.4, p=0.01). Furthermore, a moderate correlation was found between the severity of microvascular damage and the severity of skin involvement (r=0.34, p=0.001). Patients with a late pattern had an increased risk of severe skin involvement (OR 4.1, 95% CI 0.9–19.8, p=0.08). A weak correlation was found between the severity of microvascular damage and the severity of muscle involvement (r=0.24, p=0.029). Further details regarding the correlations between the severity of microvascular damage and the severity of visceral involvement for each domain of the Medsger DSS can the found in Supplementary Data Table 1.
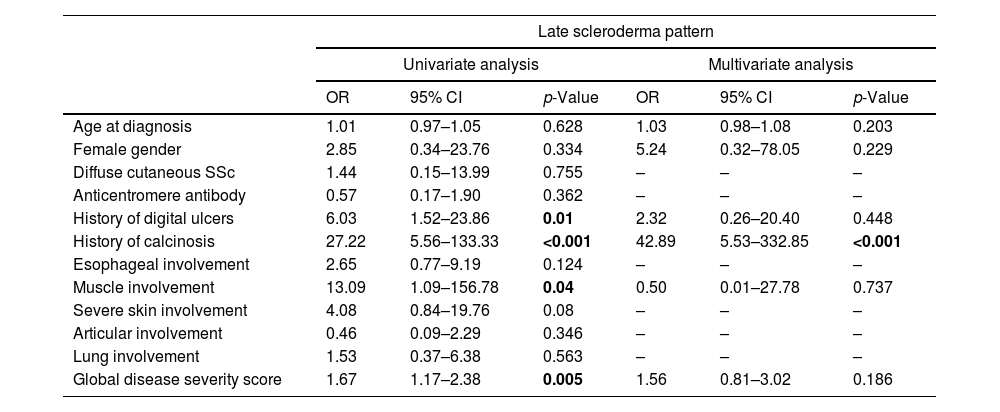
The presence of a late scleroderma pattern in NVC were predictive in univariate analysis of digital ulcers (OR 6.03, 95% CI 1.52–23.86, p=0.01), muscular involvement (OR 13.09, 95% CI 1.09–156.78, p=0.04), calcinosis (OR 27.22, 95% CI 5.56–133.33, p<0.001) and worse global disease severity score (OR 1.67, 95% CI 1.17–2.38, p=0.005). Multivariate analysis adjusted for disease duration and gender confirmed late pattern as an independent predictor of calcinosis (OR 42.89, 95% CI 5.53–332.85, p<0.001), as observed in Table 4.
Univariate and multivariate analysis to identify predictive factors for the occurrence of a late scleroderma pattern.
| Late scleroderma pattern | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age at diagnosis | 1.01 | 0.97–1.05 | 0.628 | 1.03 | 0.98–1.08 | 0.203 |
| Female gender | 2.85 | 0.34–23.76 | 0.334 | 5.24 | 0.32–78.05 | 0.229 |
| Diffuse cutaneous SSc | 1.44 | 0.15–13.99 | 0.755 | – | – | – |
| Anticentromere antibody | 0.57 | 0.17–1.90 | 0.362 | – | – | – |
| History of digital ulcers | 6.03 | 1.52–23.86 | 0.01 | 2.32 | 0.26–20.40 | 0.448 |
| History of calcinosis | 27.22 | 5.56–133.33 | <0.001 | 42.89 | 5.53–332.85 | <0.001 |
| Esophageal involvement | 2.65 | 0.77–9.19 | 0.124 | – | – | – |
| Muscle involvement | 13.09 | 1.09–156.78 | 0.04 | 0.50 | 0.01–27.78 | 0.737 |
| Severe skin involvement | 4.08 | 0.84–19.76 | 0.08 | – | – | – |
| Articular involvement | 0.46 | 0.09–2.29 | 0.346 | – | – | – |
| Lung involvement | 1.53 | 0.37–6.38 | 0.563 | – | – | – |
| Global disease severity score | 1.67 | 1.17–2.38 | 0.005 | 1.56 | 0.81–3.02 | 0.186 |
NVC is a unique, non-invasive and easy technique that allows the morphological evaluation of the nailfold capillaries.3 Its major role in rheumatology is to aid in the differential diagnosis of primary and secondary Raynaud's phenomenon. Capillaroscopic changes can be observed in several connective tissue diseases, namely in SSc, systemic lupus erythematosus (SLE), dermatomyositis, mixed connective tissue disease (MCTD), undifferentiated connective tissue disease, rheumatoid arthritis and overlap syndromes.12 In SSc, these capillaroscopic changes are specific and detectable in the vast majority of patients.4,5 It is noteworthy that “scleroderma” type capillaroscopic changes have been validated and accepted as diagnostic criterion in the current 2013 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for SSc.6
Beyond its crucial role in SSc diagnosis, NVC may also serve as a valuable indicator of disease activity and severity, as suggested by previous studies.13–15 However, most of these studies only examined specific manifestations of the disease and have not explored the association between the capillaroscopic changes and the overall disease severity.
In our study, we identified a moderate correlation between the severity of microvascular damage, as indicated by the worsening of the NVC patterns, and the global measure of disease severity, determined by the summation of the Medsger DSS for each domain. Notably, patients exhibiting a late scleroderma pattern had a significantly higher disease severity score and this pattern was predictive of a worse global disease severity score. To the best of our knowledge, only one other study, conducted by Avouac et al., has explored the correlation between the NVC changes and the global disease severity score using the Medsger DSS.16 Avouac et al. reported that a reduction in the number of capillaries and the presence of neoangiogenesis were associated with the worsening of the Medsger DSS.16 Ingegnoli et al. found that in cases of less severe organ involvement, the early or active scleroderma patterns were more commonly observed, whereas in more severe forms of the disease, the late scleroderma pattern became more prevalent.17 These findings, along with our own research, strongly emphasize the crucial role of NVC as a predictor of the extent of internal organ involvement and the disease severity.
Regarding each specific manifestation, we observed that patients exhibiting a late pattern in the NVC had a significantly increased risk of developing digital ulcers. Additionally, we identified a correlation between the severity of microvascular damage and the severity of peripheral vascular involvement. These findings are in agreement with previous reports. Avouac et al. found that a reduction in the number of capillaries and the presence of neoangiogenesis were associated with the onset of new digital ulcers, while other authors have reported that the risk of developing digital ulcers was significantly higher in patients with a late pattern.14,18,19
Regarding calcinosis, we found that patients exhibiting a late pattern in the NVC had a significantly higher risk of development of calcinosis and that the late scleroderma pattern was a predictor of calcinosis in both univariate and multivariate analyses. Calcinosis was a relatively underexplored topic in previous research. However, two previous studies, conducted by Morardet et al. and Baron et al., also found that calcinosis is independently associated with the late scleroderma pattern, and particularly, with severe capillary loss.20,21
Furthermore, we found that patients with a late pattern in the NVC had a significantly increased risk of severe skin involvement. We also identified a moderate correlation between the severity of microvascular damage and the extent of skin involvement. These findings are well-supported in the literature, as previous studies have reported that late patterns are associated with a more extensive skin involvement and a reduction in the number of capillaries may predict the progression of skin thickness.3,14,16,18
Moreover, a weak correlation between the extent of microvascular damage and the severity of muscle involvement was found in our study. Additionally, a late scleroderma pattern appears to predict muscular involvement in SSc patients. To the best of our knowledge, only one study, conducted by Smith et al., has explored the link between NVC patterns at baseline and the severity of muscle involvement, and no significant association was reported. Given the limited number of patients in this study who exhibited muscle involvement, we recommend caution in the interpretation of these results.
Contrary to previous reports, our findings did not reveal any noteworthy correlation between the severity of microvascular damage and lung involvement, including both ILD and PAH.14,15,22,23 Most of our patients with lung involvement exhibited either an active or a late scleroderma pattern, but no significant difference was found among the different NVC patterns. It's worth noting that the absence of this association may be related to the limited number of patients with lung involvement, particularly those with PAH.
Our study had some limitations that should be acknowledged. Major limitations are related to the single-centre retrospective nature of the study. Other limitation includes the small number of patients with cardiac and muscle involvement, so generalization in these cases should be done cautiously. Since we did not have any patient with renal involvement, it was not possible to assess the association with NVC patterns. Furthermore, we did not assess the potential influence of medications taken prior to the NVC, which could eventually interfere with the results of the NVC.
Further prospective studies with larger samples size are warranted, especially to investigate the possible association between capillaroscopic changes and the overall disease severity, as well as the possible associations between different organ involvement, including muscle, renal and cardiac involvement, which have been poorly studied before.
ConclusionIn our study, the overall disease severity in SSc is associated with the worsening of NVC pattern. Also, the severity of peripheral vascular involvement and extension of skin involvement was associated with worsening of NVC pattern. Additionally, the late scleroderma pattern seems to predict the presence of digital ulcers, calcinosis, muscular involvement and severe disease.
This study highlights the importance of NVC as a prognostic tool and a possible predictor of systemic visceral involvement. It may aid the physicians in risk stratification, ensuring a careful monitoring and more aggressive treatment for patients at higher risk of a severe disease.
Conflict of interestsNone to declare.