Acquired thrombotic and thromboembolic disorders may be presented initially with symptoms and signs of acute ischaemia or organ dysfunction that will lead many of these patients to seek care in the emergency department. We report a case of a 19-year-old female patient who developed catastrophic antiphospholipid syndrome (CAPS syndrome or Asherson syndrome) 6 weeks post stillbirth with an initial presentation of acute vascular occlusion. The patient was immediately operated and anticoagulated with significant improvement.
Los trastornos trombóticos y tromboembólicos adquiridos pueden manifestarse inicialmente con signos y síntomas de isquemia aguda o disfunción orgánica que derivará a muchos de estos pacientes al servicio de urgencias. Se presenta el caso de una paciente de 19 años de edad que desarrolló un síndrome antifosfolípido catastrófico (o síndrome de Asherson) 6 semanas después del parto de un feto muerto con una presentación inicial de oclusión vascular aguda. La paciente fue intervenida inmediatamente y se inició un tratamiento con anticoagulantes que supuso una mejora significativa.
In the 1980s and 1990s, some case reports emerged in the literature documenting patients with thrombotic complications, often fatal, associated with the presence of antiphospholipid antibodies. Only in 1992 for the first time, 10 patients with this unusual condition were revisited. The main characteristics of these were properly review in an attempt to define the severity and rapidity of installation and a catastrophic adjective was added to represent this variant of the antiphospholipid syndrome (APS).1 Since then, the term catastrophic antiphospholipid syndrome (CAPS syndrome or Asherson syndrome) was accepted and has been used extensively.
Case descriptionWe present a case of a 19-year-old black woman that presented to the Emergency Department with abdominal and left thigh pain. Previous medical and obstetric history included a recent stillbirth 6 weeks ago, at 36 weeks with positive lupus anticoagulant. Recent admission to Hospital due to abdominal pain, seen by Medical and Surgical team that ruled out acute pathology and was referred to Obstetrics service that discharged the patient with further follow-up. At arrival the patient was agitated and uncooperative complaining of abdominal and left thigh pain. The patient reported severe pain in her left leg accompanied by nausea, she did not have chest pain, shortness of breath, fever, chills, dysuria, constipation, or diarrhoea. The temperature was 37.1°C, the pulse was 91, and the respiratory rate 18. The blood pressure was 135/85mmHg. The patient was intermittently agitated. Examination revealed diffusely tender abdomen but not distended; the bowel sounds were normal. Examination of the extremities demonstrated absent pedal pulses on both sides, and a mild sensory loss on the left leg. Morphine sulphate, paracetamol and fluids were administered. A point-of-care ultrasound of lower legs showed the absence of a flow signal suggestive of vascular occlusion.
Initially the patient was suspected to have a sickle cell pain crisis however a reviewed of recent blood tests including electrophoresis did not show the presence of homozygous HbS or documented other hemoglobinopathies. Blood tests revealed; Hb 110g/L, WBC 13.6×109/L, Platelets 151×109/L, total bilirubin 5μmol/L, ALT 30U/L, GGT 98U/L, calcium 2.36mmol/L, phosphate 0.95mmol/L, ALP 125U/L, sodium 136mmol/L, potassium 3.4mmol/L, urea 3.0mmol/L, creatinine 83μmol/L (reference range: 0–110), AKI Stage 1, blood gases showed lactic acidosis type A. Increased reticulocytes and free haemoglobin indicated a hemolytic anaemia with scanty schistocytes on peripheral blood smear analysis. Troponin T 61, (reference range: <14) with no ST segment changes on electrocardiography.
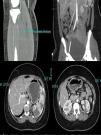
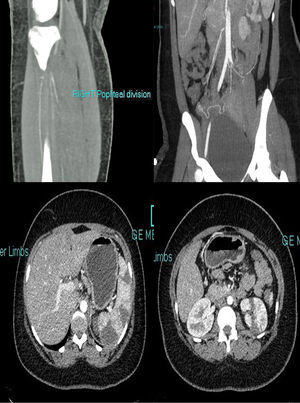
An emergency CT (Fig. 1) was requested that reported extensive fresh thrombus is noted in the left inflow, left common femoral artery and the left superficial femoral artery. Thrombus also noted in the right internal iliac, right profound and superficial femoral artery origin. Fresh thrombus noted in the right distal popliteal and trifurcation origin. Multiple splenic and left renal infarcts noted, a CT brain did not reported acute ischaemic changes. The trachea was intubated, and ventilatory assistance was begun. Vascular surgeons were requested and the patient was operated on: under general anaesthesia, a left iliofemoral embolectomy associated to a fasciotomy, a thrombus was found in the left common iliofemoral vessels. The patient was anticoagulated with warfarin.
A transthoracic echocardiogram and cardiac MRI were performed and did not detected any proximal source of emboli. Anticardiolipin IgM 9.8 MPLμ/ml, aCL IgM negative (<10), fibrinogen 5.6g/L (1.50–4.00), DRVVT positive lupus anticoagulant. An MRI 3 days after the onset showed axial FLAIR image showed right frontal borderzone infarction. Extensive autoimmune testing looking for autoimmune disorders potentially responsible for thrombotic events were all negative. Patient's clinical features were consistent with CAPS (symptoms presenting in 24h, including multiple renal and splenic infarcts, non-ST elevation myocardial infarction (NSTEMI), acute vascular thrombotic occlusion with arterial thrombi confirmed by pathology and positive lupus anticoagulant on two separate occasions 12 weeks apart) (Table 1). Intravenous hydrocortisone 600mg for 5 days was used followed by oral therapy with prednisone with an initial dose of 60mg, she was slowly tapered off prednisone and currently remains off steroid therapy. At 1 year the patient's evolution has been satisfactory, antiphospholipid tests and immunological screens has been performed and remained positive, there were no clinical or serological features of SLE. There was also no evidence of rheumatoid arthritis developing. Anticoagulation with warfarin remains on to date.
Preliminary classification criteria for catastrophic antiphospholipid syndrome.
| 1. Evidence of involvement of three or more organs, systems and/or tissues |
| 2. Development of manifestations simultaneously or in less than a week |
| 3. Confirmation by histopathology of small-vessel occlusiona |
| 4 Laboratory confirmation of the presence of antiphospholipid antibodiesb |
| Definite catastrophic antiphospholipid syndrome |
| • All four criteria present |
| Probable catastrophic antiphospholipid syndrome |
| • All four criteria, except only two organs, systems, and/or tissues involved |
| • All four criteria, except for the absence of laboratory confirmation of antiphospholipid antibodies |
| • Criteria 1, 2, and 4 |
| • Criteria 1, 3, and 4, with the development of a third event more than 1 week but within 1 month of presentation, despite anticoagulation |
This case represents a complex medical patient in a busy ED service. Upon arrival it was difficult to obtain a clear history due to her confusion state. Initially her clinical symptoms suggested an acute vaso-occlusive crisis in the context of a suspected non-diagnosed sickle cell anaemia. A bedside ultrasound showed a suspected occlusion of the left lower extremity and the blood tests were not suggestive of sickle cell disease. CT showed multiple thrombotic events and a limb-threatening thrombosis. The positive history of anticardiolipin antibodies and the recent stillbirth suggested the diagnosis of APS. The patient had a successful recovery with a final diagnosis of CPAS syndrome.
Clinical manifestations and pathological features present in patients with CAPS syndrome depend mainly on two factors: the organs affected by the thrombotic events (thrombosis extension) and systemic inflammatory response syndrome manifestations.2 The occlusion of medium or large calibre vessels is rare in the CAPS. Dysfunction of multiple organ is due to acute microangiopathy thrombosis affecting small vessels in various organs. Our patient presented with acute lower limb vascular occlusion and multiple splenic and left renal infarcts, acute confusion and NSTEMI. No previous report of life threatening limb vascular occlusion has been reported. The most significant laboratory findings include hemolytic anaemia and thrombocytopenia, hemolytic anaemia has been described in 39% of the patients with most of the cases with positive Coombs test. Coagulation tests shows characteristics of intravascular disseminated coagulation (DIC), such as prolonged prothrombin time and increase in fibrinogen degradation products. Blood smear may reveal schistocytes, that are usually scanty, unlike the abundant numbers seen in patients with thrombotic thrombocytopenic purpura, the meaning of schistocytes is still uncertain. 68% of patients in the two main series of cases show antiphospholipid antibodies. The diagnosis of CAPS can be challenging, and sometimes the differential diagnosis cannot be narrowed to a single disease during the acute period. Thus, continuous assessment of patients is warranted.
An interface of HELLP Syndrome and CAPS exists. A series of 15 cases of CAPS, which appeared during pregnancy or the puerperium were described.3 HELLP syndrome occurred in eight (53%) of the patients. Mortality from the obstetric catastrophic CAPS cohort was extraordinarily high, with 46% (seven out of 15) of the mothers and 64% (seven out of 13) of the neonates succumbing to the disease process. Our reported case was diagnosed in the puerperium, although no previous diagnosis of preeclampsia or HELLP syndrome was established. It is important to consider the possibility of the development of CAPS in those patients with signs of HELLP syndrome and multiorgan failure during pregnancy or puerperium, especially in those patients with previous history of abortions and/or thrombosis.
Regarding the long term outcome of CAPS survivors; an analysis of 130 patients showed that 66% of patients who survive an initial event remained symptom free with anticoagulation during an average follow up of 67.2 months.4 Treatment with intensive anticoagulation, plasma exchange, and corticosteroids appears beneficial, but no controlled trials have been performed. Intravenous immunoglobulin may be of some benefit, and rituximab or cyclophosphamide may be considered in selected cases, especially in SLE-associated CAPS.
ConclusionsPatients with hypercoagulable states presents in EDs with several symptoms and signs including life-threatening conditions,5 the reported case is an example of the complexity of these complex pathologies, therefore emergency physicians should be familiar with the diagnosis and start immediately lifesaving therapies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare they have no conflicts of interests.