To assess the positioning that patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), ankylosing spondylitis (AS) and their proxies give to their diseases.
MethodsSubjects completed a self-administered questionnaire to rank 11 diseases from “worst” to “least bad”. Then they defined the “worst” disease and ranked 10 diseases from highest to lowest importance from a list including “my rheumatic disease/my relative's disease”. The lists of the included diseases represented the mindshare from a sample of healthy adults.
ResultsThere were 570 respondents (104 SLE, 99 RA, 82 AS, and 285 proxies). Rheumatoid arthritis was considered the third-worst disease (recoded ranking first by 41% of patients and 43% proxies, second by 49% and 44%, and third by 10% and 13%). A disease that kills was the preferred definition for the worst disease. “My disease/my relative's disease” was ranked fourth in importance (first by 41% of patients, second by 38%, and third by 21%). Rankings were not associated with age, schooling, disease duration, or setting.
Discussion and conclusionsMost respondents ranked their own disease considerably lower than other non-rheumatic conditions.
Evaluar el posicionamiento que pacientes con lupus eritematoso sistémico (LES), artritis reumatoide (AR), espondilitis anquilosante (EA) y sus acompañantes dan a sus enfermedades.
MétodosLos participantes completaron un cuestionario para clasificar 11 enfermedades de «peor» a «menos mala». Luego definieron la «peor» enfermedad y el ranking de 10 enfermedades de una lista que incluía «mi enfermedad reumática/de mi familiar». Las listas de enfermedades incluidas representaron la «conciencia de marca» de un grupo de adultos sanos.
ResultadosHubo 570 encuestados (104 LES, 99 AR, 82 EA y 285 acompañantes). La AR se posicionó como la tercera peor enfermedad (en primer lugar, por el 41% de pacientes, segundo por el 49% y tercero por el 10%). La definición preferida para «peor» enfermedad fue aquella que mata. «Mi enfermedad reumática/de mi familiar» fue la cuarta más importante (primer lugar por el 41% de pacientes, segundo por el 38% y tercero por el 21%). El posicionamiento no estuvo asociado con edad, escolaridad, duración de la enfermedad ni centro de atención.
Discusión y conclusionesLa mayoría de los encuestados calificaron su enfermedad reumática más abajo que otros padecimientos no reumáticos.
Defining the importance of a disease depends on many factors, including the perspective (e.g., cost, public policies, politics, or social support requirements). Assigning “importance” can be relevant for many reasons. For example, at the macro level, it can affect resource allocation, organization of health systems, and public policies. At the individual level, it affects disease self-management, and the interactions with a social network or using social capital.
Intuitively, it seems likely that a patient with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), or ankylosing spondylitis (AS) would consider their own disease the most important. In this context, the perception of their disease's “importance” among patients can affect variables that are considered powerful health determinants1 and predictors of disease-related outcomes.2–4 It is therefore worth exploring the “importance” that patients and their relatives (proxies) give to the illnesses they are facing.
For this study, we drew on some marketing concepts. The mindshare describes the diseases that people have in their minds, or the diseases for which there is high awareness, as this affects attitudes. Most people can only remember a certain number of diseases, and top-of-mind awareness refers to the disease that is considered first by people when they think about which diseases ‘matter’. The mindshare and top-of-mind are useful for connecting customers (patients) and a service (rheumatologists), which lays the foundation for positioning. Positioning5 is the place that a disease occupies in the minds of patients, proxies, or the broader population, and how it is distinguished from the other diseases. This is part of the interpretative structures. We defined interpretative structures as how society and patients perceive, categorize and give meaning to diseases and then act on that meaning.6 Indirect data suggest that the mindshare and positioning of rheumatic diseases is low. For example, in North America, web searches for turmeric outnumber those for all disease-modifying anti-rheumatic drugs combined, and those for arthritis are far fewer than for hepatitis C or breast cancer in the US and Mexico.7
This study aimed to assess where patients with SLE, RA, or AS and their proxies position these diseases in a mindshare list of worst and most important diseases.
Patients and methodsThis was a cross-sectional study of a convenience sample of patients with either SLE,8 RA,9 or AS,10 and their proxies who attended a private- or a public, secondary-level outpatient rheumatology clinic in Guadalajara, Mexico during a 9-month period. Proxies were adults who accompanied patients to the appointment. Patients and their proxies were invited to separately and simultaneously complete an anonymous questionnaire before meeting the rheumatologist.
The self-administered questionnaire contained four demographic questions and three study questions. The first study question required participants to rank a list of 10 diseases plus RA from “worst” to “less bad”(“From this list of diseases, please rank with the number 1 the one you consider to be the worst, with the number 2 the one that you consider to be next, and so on”). The second question asked participants whether their decision to rank the “worst disease” in question one was based on the disease's ability to cause death, to cause pain, or to result in disability (“In relation to the previous question, how do you define “worse”? One that kills; one that hurts a lot; one that disables.”). The third question required participants to rank diseases by importance, and the answer options included a list of nine diseases, plus “my rheumatic disease” for patients and “my relative's disease, which is called...” for proxies. The diseases were listed vertically in random order.
The diseases listed as answer options for questions one and three were the “mindshare” diseases obtained from a convenience sample of 50 self-declared healthy people not related to the study participants. These 50 people were asked to name 10 diseases that they considered the “worst” diseases and 10 that they considered the “most important”. The sampling stopped upon reaching saturation (repetition) of the 10 “worst” and 10 “most important” diseases. The “worst” diseases were diarrhea, diabetes, flu, high blood pressure, measles, breast cancer, AIDS, scabies, epilepsy and urinary tract infection. All 10 diseases were included because the last two options were tied, giving a total of 11 conditions with RA. The “most important” diseases were high blood pressure, diabetes, cancer, stroke, kidney failure, gastritis, typhoid fever, hepatitis, and myocardial infarction, giving a total of 10 after adding “my rheumatic disease/my relative's disease”.
Statistics and reportingThe working hypothesis was that RA and “my own disease” would be top of mind (the first three places among the 10 or 11 options) among the surveyed patients and proxies. We did not attempt to make between-group comparisons because participants were not selected to be comparable. However, we assessed differences between rankings and settings, schooling and disease duration using chi-squared, the t-test for independent samples and one-way ANOVA with post hoc Scheffé test for multiple comparisons, when appropriate.
Only questionnaires that were at least 97% complete were included in the analysis. Missing values were not substituted and the results for each item are reported as absolute totals (raw values). The rankings of the “worst” and “most important” lists of diseases were analyzed as crude rankings (1–11 and 1–10) and as recoded rankings, where 1st to 3rd place=1, 4th to 7th=2, and 8th to 10th or 11th=3. Significance was set at p≤0.05.
The study was approved by the Comité de Bioética of the Unidad de Investigación en Enfermedades Crónico-Degenerativas number R-1307. The questionnaire responses did not contain sensitive information that could identify any participants. The participants expressed verbal consent to participate.
ResultsAll invited patients and proxies agreed to participate. There were 581 respondents, but 11 were excluded for not completing it. In total, 570 (98%) people completed the questionnaire. Table 1 shows selected characteristics of the respondents. Overall, 285 were patients (104 with SLE, 99 RA and 82 AS) and 285 were proxies.
Selected characteristics of the 570 respondents by study group.
| Variableb | SLE | RA | AS | |||
|---|---|---|---|---|---|---|
| Patients, n=104 | Proxies, n=104 | Patients, n=99 | Proxies, n=99 | Patients, n=82 | Proxies, n=82 | |
| Age, yrs.±SD | 35±13 | 40±15 | 50±14 | 42±16 | 40±14 | 43±14 |
| Female, n (%) | 66 (63) | —a | 70 (71) | —a | 41 (50) | —c |
| Schooling, n (%) | ||||||
| 1th–6th | 15 (15) | 27 (26) | 35 (36) | 23 (24) | 10 (13) | 27 (33) |
| 7th–9th | 34 (33) | 26 (25) | 23 (23) | 35 (35) | 28 (34) | 17 (21) |
| 10th–12th | 31 (30) | 36 (35) | 9 (9) | 15 (15) | 14 (17) | 21 (26) |
| >12th | 24 (22) | 15 (14) | 32 (32) | 26 (26) | 30 (36) | 17 (20) |
| Disease duration, yrs±SD | 7±7 | — | 13±10 | — | 10±9 | — |
| Worst disease, n (%) | ||||||
| It kills | 52 (50) | 52 (50) | 30 (30) | 42 (43) | 32 (39) | 47 (57) |
| It hurts a lot | 26 (25) | 27 (26) | 14 (14) | 20 (20) | 15 (18) | 9 (11) |
| It disables | 26 (25) | 25 (24) | 55 (56) | 37 (37) | 35 (43) | 26 (32) |
| Setting, private, n (%) | 18 (17) | 23 (17) | 37 (37) | 37 (37) | 39 (48) | 39 (48) |
| Data entries lost, nc | 10 | 9 | 3 | 5 | 3 | 4 |
Rounded numbers.
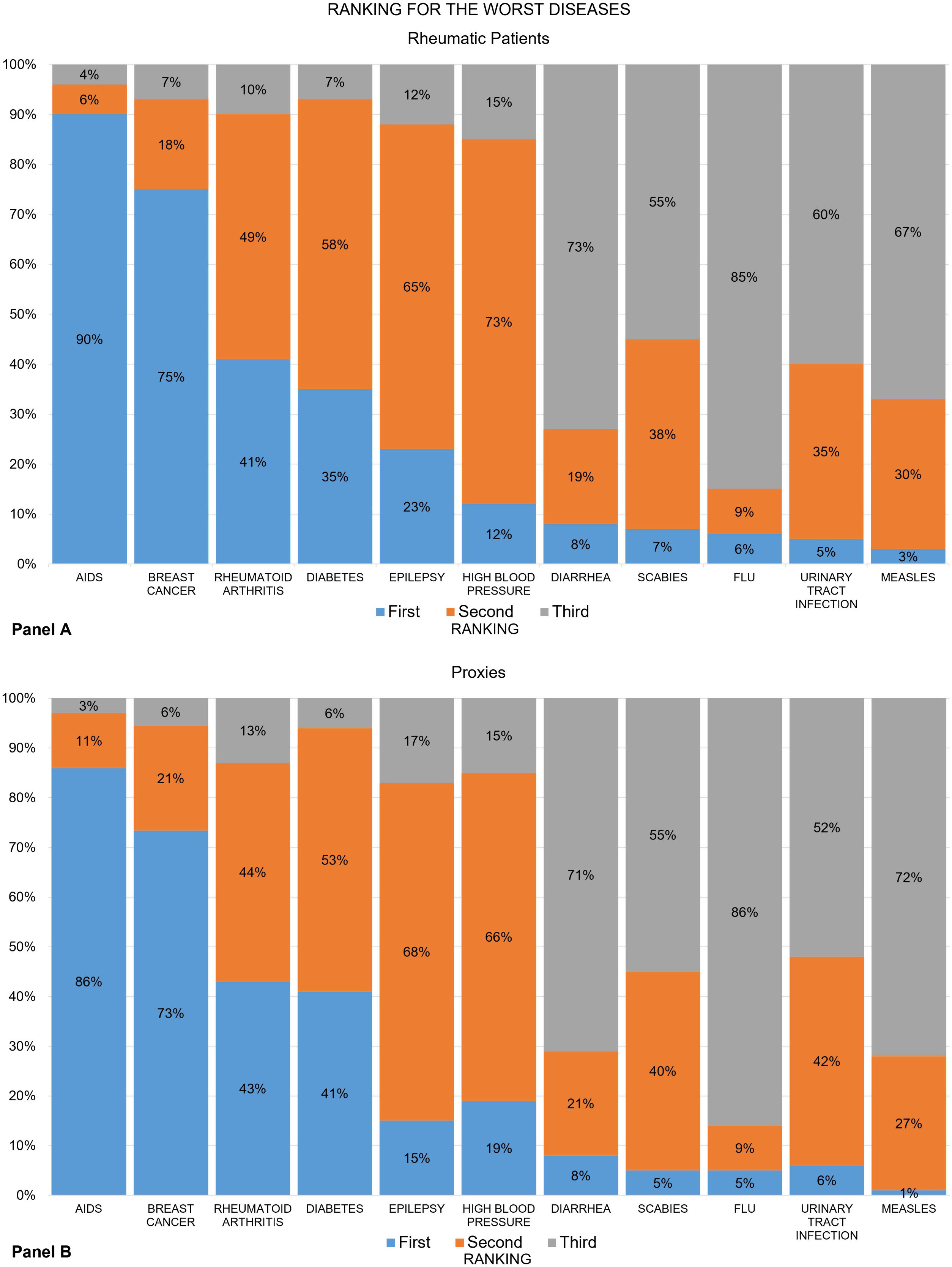
Across all groups, the least used definition by patients and proxies for the worst disease was “it hurts a lot”. The frequency of participants who opted for “it disables” varied between the study groups, and was highest (56%) for patients with RA and lowest (24%) for the SLE-proxy group (Table 1).
No differences in the mindshare ranking lists were found among the patients with SLE, RA, or AS, or their proxies, so the results are reported in two groups for simplicity: all patients and all proxies. Results are reported as recoded rankings.
Figures show the frequency distribution of the rankings for the “worst” (Fig. 1) and the “most important” diseases (Fig. 2) among the group of rheumatic patients (panels A) and their proxies (panels B). Overall, nearly 9 out of 10 patients ranked AIDS as the worst disease on the list, and RA was placed third. The recoded ranking for RA was first for 41% of patients, second in 49% and third in 10% (panel A). These numbers varied slightly across groups. For example, RA ranked first in 51% of the RA group, 45% of the AS group, and only 28% of the SLE group (data not shown). The proxy groups showed similar results (panel B).
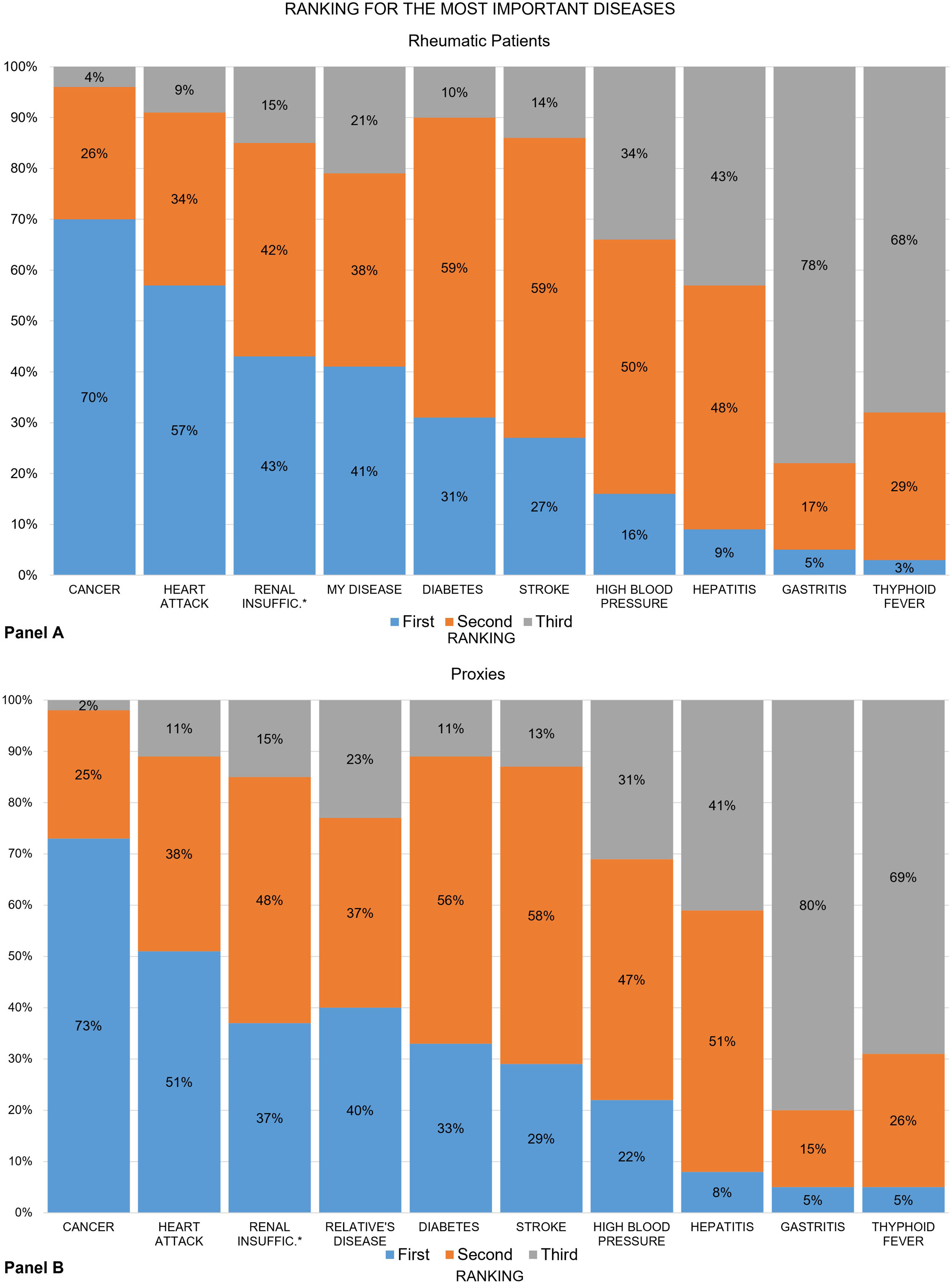
Seven out of 10 patients ranked cancer as the “most important” disease, and “my disease” was ranked fourth. The recoded ranking of “my disease” was first among 41% of patients, second in 38%, and third in 21% (Fig. 2, panel A). These numbers also varied slightly across groups (data not shown). The proxy groups showed similar results in almost everything (panel B), except in RA, where 7% fewer proxies ranked their relative's disease first (42% vs. 35%).
Rankings were not significantly associated with age, schooling, disease duration or setting (private vs. public) (data not shown).
DiscussionThis exploratory study assessed the positioning of two concepts—RA and “my own disease/my relative's disease”. Patients and proxies were asked to rank two lists of diseases from the mindshare of the worst and most important diseases among healthy individuals. It is relevant to study positioning as its components may be potentially modifiable, impacting the outcomes.
The main finding is that both RA's positioning and “my own disease/my relative's disease” within the “most important” list were low, even among those with this disease and their proxies. Just 4 in 10 patients ranked their own disease as the most important, and 20% rated it as the least important. The poor positioning was also similar within the list of “worst” diseases in all studied groups, although many of the patients had had the disease for years, had different schooling levels, and were in private or public clinics.
There are several plausible explanations for these findings. It is possible that the social perception of diseases in general, and rheumatic diseases in particular, does not change after disease onset. Indeed, many individuals actively avoid potentially helpful information as a way to protect their beliefs.11 Information avoidance is pervasive and may be related to perceived actionability,11 which in turn may be closely related to self-efficacy or learned helplessness. It is also possible that our patients and their representatives have a conception of disease where cultural beliefs, traditional practices and social relationships are integrated differently to mainstream medicine.12 However, the opposite is worth considering. Respondents may perceive that they are in control of their disease, so they give more importance to other diseases which, were they to suffer from, they would not have the same control. It would be the opposite of learned helplessness, which would be interesting to study in further research.
There are several limitations worth considering. First, this is a cross-sectional study, precluding causality assumptions. Second, the sample may be biased because participants were all patients with companions at the time of the survey. Third, the two lists of diseases were based on healthy people's mindshare, so these results might vary if the lists were different in content or in the order in which the options were listed. Fourth, the way we assessed positioning may not reflect the full spectrum for a patient or family member. Indeed, the definition of “disease” has been debated for decades, even among clinicians.12–14 Fifth, we did not find any similar studies for comparison, but indirect data suggest that our figures are probably generalizable. For instance, there were 4–60 times more internet searches for turmeric than the most-searched biological disease-modifying anti-rheumatic drug in Canada, USA and Mexico.7 Similarly, there are more searches for hepatitis and breast cancer than arthritis in the USA and Mexico.7 Sixth, social perception on specific health issues may have changed since our survey because of the COVID-19 pandemic.
ConclusionsIn conclusion, respondents were dealing with a chronic, systemic and potentially disabling disease, but the majority still ranked their condition well below others. This may have detrimental consequences for their disease self-management and behavioral variables. Future studies may wish to consider whether repositioning strategies used in marketing could change some of these poor-outcome predictors.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Availability of data and material (data transparency): data available upon request.
Conflict of interestNone declared.