Sarcoidosis is a chronic granulomatous disease characterized by non-caseating granuloma. The conventional chest X-ray (CXR) has important role in the diagnosis, staging and follow-up of disease. Computed tomography (CT) is a second-line imaging method used to determine the extent, complications and differential diagnosis of sarcoidosis.
ObjectivesTo determine the role of CXR in the early diagnosis and staging of sarcoidosis and to compare with CT imaging.
MethodsOne hundred and nine sarcoidosis patients followed at a single center were included in the study. Demographic, radiological, and clinical data of 81 patients were obtained from a total of 109 patients, and the record data of these 81 patients were evaluated. Patients who could not be reached for all tests were excluded from the study. CXR and CT imaging taken at diagnosis were evaluated retrospectively independently from two radiologists and one rheumatologist.
ResultsAmong 109 patients, eighty-one patients CXR and CT imaging taken at the same center has been reached. Among 81 sarcoidosis patients 23 (28.4%) were male, 58 (71.6%) were female. The mean patients age was 46.4 years and the mean disease duration was 3.8 years. CXR is regarded as normal at diagnosis in 30 patients (37%), while all of these patients had findings consistent with sarcoidosis on CT imaging. CT imaging are more superior than CXR in the early diagnosis and staging of sarcoidosis (p=0.001). Also CT imaging is more superior for detection of disease extent and complications.
ConclusionsIn this study, we observed that CT imaging outperforms CXR in terms of early detection and staging of sarcoidosis. The use of CT imaging is important for early diagnosis and staging of sarcoidosis. The low performance of CXR is a condition that requires the discussion of this method. Multicenter prospective study is needed in this regard.
La sarcoidosis es una enfermedad granulomatosa crónica caracterizada por un granuloma no caseificante. La radiografía de tórax convencional (CXR) tiene un papel importante en el diagnóstico, estadificación y seguimiento de la enfermedad. La tomografía computarizada (TC) es un método de imagen de segunda línea que se utiliza para determinar la extensión, las complicaciones y el diagnóstico diferencial de la sarcoidosis.
ObjetivosDeterminar el papel de la radiografía de tórax en el diagnóstico temprano y la estadificación de la sarcoidosis y compararlo con la tomografía computarizada.
MétodosSe incluyeron en el estudio 109 pacientes con sarcoidosis seguidos en un solo centro. Se obtuvieron datos demográficos, radiológicos y clínicos de 81 sujetos de un total de 109 pacientes, y se evaluaron los datos de registro de estos 81 individuos. Los pacientes que no pudieron ser contactados para todas las pruebas fueron excluidos del estudio. Las imágenes de CXR y CT tomadas en el momento del diagnóstico fueron evaluadas retrospectivamente de forma independiente por 2 radiólogos y un reumatólogo.
ResultadosDe un total de 109 pacientes se han obtenido imágenes de CXR y CT, tomadas en el mismo centro, de 81 individuos. De esos 81 pacientes con sarcoidosis 23 (28,4%) eran hombres y 58 (71,6%) eran mujeres. La edad media de los pacientes fue de 46,4 años y la duración media de la enfermedad fue de 3,8 años. La CXR se considera normal en el momento del diagnóstico en 30 pacientes (37%), mientras que todos estos pacientes tenían hallazgos consistentes con sarcoidosis en la TC. La TC es superior a la radiografía de tórax en el diagnóstico temprano y la estadificación de la sarcoidosis (p=0,001) y en la detección de la extensión de la enfermedad y las complicaciones.
ConclusionesEn este estudio observamos que la TC supera a la radiografía de tórax en términos de detección temprana y estadificación de la sarcoidosis. El uso de imágenes por TC es importante para el diagnóstico precoz y la estadificación de la sarcoidosis. El bajo rendimiento de CXR es una condición que requiere la discusión de este método. Son necesarios estudios prospectivos multicéntricos al respecto.
Sarcoidosis is a chronic granulomatous disease characterized by non-caseating granuloma formation. Although the pathogenesis is still not clearly yet, activation of the cellular immune system and nonspecific inflammatory response can occur with the effects of some genetic and environmental factors.1 Generally, it is more common among women and mostly occurs between 20 and 40 years of age, although a second peak has been reported over 50 years old. Sarcoidosis is a chronic granulomatous disease which can present with various clinical manifestations. The disease presents most often with bilateral hilar lymphadenopathy, pulmonary infiltrates, and skin and eye lesions.2 The diagnosis of sarcoidosis is not standardized, but is based on three major criteria: a compatible clinical presentation, the finding of non-necrotizing granulomatous inflammation in one or more tissue samples, and the exclusion of alternative causes of granulomatous disease.3 In nearly 90% of sarcoidosis patients, mediastinal and hilar lymph nodes as well lungs are affected. Abnormal chest imaging findings are often the primary clinical characteristics of pulmonary sarcoidosis. Although the chest radiography (chest X-ray, CXR) is a gold standard for diagnosis and staging of pulmonary sarcoidosis, its diagnostic accuracy is only 50% (Table 1).4 Its advantages include its wide availability, low cost and low radiation exposure. Although the CXR is recommended imaging method for detection of pulmonary sarcoidosis, it is insufficient to detect small lung nodules, thin patchy lesions, mediastinal lymph node enlargement, early parenchymal and pleural involvement.5 The sensitivity of X-ray in detecting these thoracic abnormalities in sarcoidosis can vary. Generally, chest X-rays are useful for identifying initial signs of the disease, such as hilar lymphadenopathy and pulmonary infiltrates. However, more advanced imaging techniques like high-resolution computed tomography (HRCT) are often required to assess the extent of the disease, identify subtle changes, and differentiate sarcoidosis from other conditions with similar presentations. Computed tomography (CT) has a higher resolution and provides clear views of the pulmonary, pleural and mediastinal pathologies as well successfully used to examine interstitial lung diseases and small lung lesions.6 According to the 1999 ATS/ERS/WASOG guidelines which are still valid, there are three indications to perform a CT scan: (a) if there are no lesions in the X-ray, but there is a clinical suspicion of sarcoidosis; (b) if there are atypical clinical or radiological symptoms; and (c) in order to diagnose possible complications.7 Despite these guidelines recommendations, the apparent benefits of CT over CXR in diagnosis, staging and prognosis of sarcoidosis were reported recently.8
Scadding staging scale.
| Scadding stage | Findings (CXR) | % of patients at presentation | Resolution in untreated patients |
|---|---|---|---|
| 0 | Normal | – | – |
| I | Lymph node enlargement | 5–15% | 50–90% |
| II | Lymph node enlargement+parenchymal changes | 45–65% | 30–70% |
| III | Parenchymal changes only | 30–40% | 10–20% |
| IV | Fibrosis | 5% | 0% |
CXR: chest X-ray.
The aim of this study is to determine the role of CXR in the early diagnosis and staging of sarcoidosis and to compare its performance with CT imaging.
Material and methodOne hundred and nine sarcoidosis patients followed-up in a single rheumatology center were retrospectively evaluated. Demographic, radiological, and clinical data of 81 patients were obtained from a total of 109 patients, and the record data of these 81 patients were evaluated. Patients who could not be reached for all tests were excluded from the study. Sarcoidosis diagnosis were made through clinical, laboratory, imaging and histopathological investigations. Demonstration of non-caseating granulomas were shown on pathological specimen. Other factors (tuberculosis, bacterial and fungal infections) that may cause granulomatous diseases were ruled out. Laboratory investigations were performed; routine biochemistry, acute phase reactants (erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)), serum ACE, calcium and hydroxy-vitamin D3 levels were checked. Conventional X-ray (CXR) and thorax computed tomography (CT) were performed for diagnosis and staging of sarcoidosis. Both conventional CXR and CT imaging that was taken at the time of diagnosis were retrospectively reviewed independently by two radiologists and one rheumatologist. The radiological diagnosis and staging results among the three specialists were discussed until a consensus was achieved. Radiological stages determined from CXR imaging was compared with that determined from CT examination.
Statistical analysisData was analyzed by the Statistical Package for the Social Sciences (SPSS) version 20.0, software for Windows (SPSS, Chicago, IL, USA). Cross tables were used in analysis of data and Chi-square and Fisher's exact test analyses were performed where appropriate. The data are given as frequency and percentages. The statistical significance threshold was taken as 0.05.
ResultsAmong 109 patients, eighty-one patients CXR and CT imaging taken at the same radiology center has been reached. Demographic, radiological, and clinical data of 81 patients were obtained from a total of 109 patients, and the record data of these 81 patients were evaluated. Patients who could not be reached for all tests were excluded from the study. Among 81 sarcoidosis patients 23 (28.4%) were male, 58 (71.6%) were female. The mean patients age was 46.4 years and the mean disease duration was 3.8 years (Table 1). As for system and organ involvement; arthritis was seen in 62 (76.5%), Löfgren's syndrome in 13 (16%), erythema nodosum in 32 (39.5%) patients, uveitis in 10 (12.3%) patients, myositis in 1 (1.2%) patient, and neurosarcoidosis in 1 (1.2%) patient. In laboratory tests, increased serum levels of angiotensin-converting enzyme (ACE) were detected in 42 (51.8%) patients, serum calcium in 7 (8.6%) patients, and serum vitamin D3 in 1 (1.2%) patient. As for the acute phase reactants, increased C-reactive protein (CRP) level was detected in 38 (46.9%) patients and increased erythrocyte sedimentation rate (ESR) in 41 (50.6%) patients. Among 81 patients, CXR is regarded as normal at diagnosis in 30 (37%) patients, while all of these patients had findings consistent with sarcoidosis on CT imaging. Eighty-one patients which have findings consistent with sarcoidosis on CT examination were as follows: stage 1 (bilateral hilar lymphadenopathy) in 52 (64.2%) patients, stage 2 (bilateral hilar lymphadenopathy+pulmonary infiltrate) in 20 (24.6%) patients, stage 3 (only pulmonary infiltrate without hilar lymphadenopathy) in 6 (7.4%) patients, and stage 4 (pulmonary fibrosis) in 3 (3.7%) patients with sarcoidosis (Table 2). Regarding disease stages, CT imaging detected more patients with stage 1 and stage 2, compared with other stages of disease (p=0.02, p=0.04 respectively) (Table 3). When compared with CXR, CT imaging was more superior in the detection of early diagnosis and stage determination of sarcoidosis (p=0.001). One patient had pleural involvement and another one had suspected pulmonary hypertension (PH) detected on CT imaging while CXR was normal in these patients both. CT imaging was more superior for early detection of disease extent and complications, such as pleural involvement, PH, micronodules and active alveolitis. The most frequent findings detected on CT examination were bilateral symmetrical hilar and mediastinal lymphadenopathy, micro and macronodules, located along bronchovascular bundles, interlobular septi, interlobar fissures, and in the subpleural region. In patients with stage 4 the features of alveolitis/fibrosis including “ground-glass” and “honeycombing”, architectural distortion, and traction bronchiectasis were seen. Most frequently, lesions in sarcoidosis demonstrate predilection to the upper and middle fields. There were not found correlation between CT findings and lung function tests (p=0.45).
Demographic, clinical and laboratory features of sarcoidosis patients.
| Features | Patients, n=81 (%) |
|---|---|
| Age, mean, years | 46.4 years |
| Disease duration, mean, years | 3.8 years |
| Sex (women/men) | 58 (71.6%)/23 (28.4%) |
| Arthritis | 62 (76.5%) |
| Löfgren's syndrome | 13 (16%) |
| Erythema nodosum | 32 (39.5%) |
| Bone lesion | 1 (1.2%) |
| Uveitis | 10 (12.3%) |
| Myositis | 1 (1.2%) |
| Neurosarcoidosis | 1 (1.2%) |
| Penile mass | 1 (1.2%) |
| Parotid involvement | 1 (1.2%) |
| Elevated serum ACE level | 42 (51.8%) |
| Elevated serum calcium level | 7 (8.6%) |
| Elevated serum D3 level | 1 (1.2%) |
| Increased CRP | 38 (46.9%) |
| Increased ESR | 41 (50.6%) |
Abbreviations: ACE: angiotensin-converting enzyme; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
Sarcoidosis is an important condition for rheumatologists to be familiar with due to its systemic nature and potential for significant organ involvement. It is a chronic inflammatory disorder characterized by the formation of granulomas in various organs, most commonly affecting the lungs and lymph nodes. However, it can also impact other organs such as the skin, eyes, heart, liver, and nervous system.9 The main pattern involvement of disease is bilateral ankle joints, but it may also involve the small joints of the hands, knee and sacroiliac joints.10 The importance of disease may increase when it presented with internal organ involvement such as lungs, brain and heart. In such situation the differential diagnosis should be done with connective tissue diseases and vasculitis.11,12 Rheumatologists play a crucial role in recognizing these systemic manifestations and differentiating sarcoidosis from other conditions with similar symptoms.13 CXR is often the initial imaging modality used to evaluate pulmonary sarcoidosis. It can reveal bilateral hilar lymphadenopathy, which is a characteristic finding in sarcoidosis. Additionally, CXR may show parenchymal opacities, particularly in the upper lung zones. The presence of these findings can raise suspicion for sarcoidosis and prompt further evaluation.
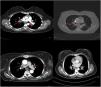
CXR-based staging of sarcoidosis was developed before the introduction of CT, and for decades, it has been the gold standard for staging, follow-up and prognosis of pulmonary sarcoidosis.14 It was preferential method because of low price, low radiation exposure and wide use as well. However, the intensity of symptoms, the presence of extrapulmonary disease, the results of pulmonary function tests, and the necessity of treatment are not well correlated with CXR.15 CXR is insufficient to portray small parenchymal abnormality in early stages of the disease, and small mediastinal and hilar lymphadenopathies. The study showed that there was no correlation between lung function tests (FVC, FEV1 and DLCO) and CXR imaging and it is not a reliable test to detect pulmonary exacerbations of sarcoidosis.16 CT scans are more sensitive than CXR in detecting lung parenchymal changes in sarcoidosis. High-resolution CT (HRCT) can reveal ground-glass opacities, nodules, and peribronchovascular thickening. CT can help in assessing disease extent and severity, guiding treatment decisions, and monitoring disease progression. Unlike X-ray scans, some CT lesions demonstrate a relationship with pulmonary function tests.17 Drent et al. demonstrated correlations between respiratory functional tests and CT features such as thickening of the bronchovascular bundle, intraparenchymal nodules, septal and non-septal lines, or focal pleural thickening.18 Zappala et al. have suggested that compared with CXR presentations, CT scan appear to be more consistent with pulmonary functional changes.19 CT is significantly more sensitive than CXR regarding detection of early sarcoidosis lesions, which may help us for early decision treatment. This is true even in stage 1/2, which is linked with a favorable long-term prognosis. Despite these facts, the predictive significance of CT findings has not yet been subjected to a significant amount of research. Even among chest radiologists, there is considerable heterogenicity and subjectivity in the interpretation of CXR. Baughman et al. evaluated the discrepancy between two chest radiologists initial assessments of Scadding staging using data from a clinical trial.20 Overall, the authors discovered only fair interobserver concordance, and with regards to the presence of fibrosis, they found only fair interobserver concordance. They also mentioned that it was hard to tell if a patient was at stage II or stage III because chest radiologists could not agree on the appearance of hilar lymphadenopathy. Zhang et al. compared the chest CT and CXR imaging for staging of pulmonary sarcoidosis using clinical records of 227 sarcoidosis patients.21 They reported that overall, 50.2% patients showed discordant sarcoidosis stage between CXR and CT, which findings support the results from our present study. The primary reason for inconsistent stage between CXR and HRCT was failure to detect mediastinal lymph node enlargement in the shadow of the heart in CXR and small nodules because of the limited resolution of CXR. Also they found more patients with pleural involvement detected by CT (25.6%) compared with CXR (7.5%). In addition, the authors recommend new staging criteria for pulmonary sarcoidosis according to CT imaging, not CXR. Russo et al. investigate the sensitivity and specificity of different chest imaging for sarcoidosis screening in patients with cardiac presentations.22 While the CXR was suboptimal as a screening test, in contrast CT and cardiac/thorax MRI had excellent sensitivity. CT has the highest specificity among imaging modalities. Sarcoidosis primarily affects the upper lung zones, and granuloma are predominantly spread along lymphatics, it is more difficult to assess these regions using CXR and when assessing lesions of this kind, CT is preferable method. CT may also distinguish confluent granulomas, which have a peculiar imaging result known as the “galaxy sign,” which resembles a star cluster when viewed through a telescope.23 CT also may help in the differential diagnosis of sarcoidosis with lymphangitic carcinomatosis, which can present with a nodular perilymphatic pattern and septal thickening. Various studies have demonstrated the superiority of CT in detecting lesions corresponding to fibrosis and in diagnosing more advanced stages of the disease.24 CXR scans revealed fibrosis in 5–10%, whereas CT scans resulted in such changes being described in as many as 20–50% of patients. In fibrotic stage, the presence of “honeycombing” and traction bronchiectasis is associated with worse prognosis, and a larger degree of fibrotic alterations detected on CT scan is known to be predictive of mortality.25 Our research results align with previous studies in the literature. In this investigation, we observed that CT imaging outperforms CXR in terms of early detection and staging of sarcoidosis (Fig. 1). The predominant stages identified through CT examination, which were not visible on CXR, were stage 1 and stage 2 (Fig. 2). Furthermore, CT imaging provided valuable insights into pleural involvement, early alveolitis, and pulmonary hypertension.25 Nevertheless, our study does have certain limitations. Firstly, the data we used was confined to a single center and involved a relatively small number of patients, which restricts the generalizability of our findings to all sarcoidosis patients. Another limitation is that our study population consisted solely of individuals of Caucasian origin, and it is widely acknowledged that disease phenotypes may exhibit significant racial differences. Consequently, it is essential to replicate our findings in a group of African-Americans to ensure their broader applicability. Furthermore, had we enrolled patients consecutively and conducted a prospective evaluation of CXR and CT scans with a larger number of readers, it would have likely minimized potential biases in the results. Despite these limitations, it is worth noting that our findings align closely with those reported in existing literature on the subject.
The current standard clinical practice and guidelines advocate commencing the evaluation of sarcoidosis patients with CXR scans. However, several studies have demonstrated that CXR alone is inadequate for early diagnosis, differential diagnosis, treatment monitoring, and prognostic assessment of the disease. Our study also supports this notion, as we found that CT imaging exhibited significantly higher superiority than traditional CXR in detecting sarcoidosis. Considering the limitations of CXR and the superiority of CT imaging, it is imperative that we reevaluate the conventional use of CXR in the diagnostic and monitoring process. Its relatively poor sensitivity and specificity may lead to missed or misdiagnosed cases. To gain a more comprehensive understanding of the issue, it is crucial to conduct multicenter prospective studies.
FundingNone declared.
Conflict of interestThe authors have no conflicts of interest to declare.