Systemic lupus erythematosus (SLE) is a multiorganic autoimmune disease with heterogeneous clinical manifestations and unpredictable disease course.1 Although the prognosis and survival of SLE patients have dramatically improved, the treatment of severe multiorganic affectation remains as a therapeutic challenge.1,2 Severe nephritis, transverse myelitis and massive pulmonary hemorrhage are three of the most serious manifestations of SLE. These conditions are associated with high morbidity and mortality, including those events that are consequence of the immunosuppressive therapy.1–4
Different abnormalities of T and B lymphocytes are involved in the pathogenesis of SLE.5–9 B lymphocytes synthesize several cytokines involved in the pathogenesis of SLE (including IL-10), have an important role as antigen presenting cells, and are the source of the different auto-antibodies detected in these patients.10,11 Therefore, B lymphocytes have been considered as a good therapeutic target in SLE.12,13 Rituximab is a chimeric monoclonal antibody, with human IgG1 and kappa light chain constant domains, and mouse variable regions from a mouse hybridoma producing an anti-human CD20 antibody.14,15 CD20 is expressed by immature and mature B lymphocytes, but not by early B cell precursors or plasma cells. This biological agent causes B-cell depletion in vivo, inducing complement and antibody dependent cellular toxicity as well as antibody mediated apoptosis.16 Interestingly, administration of Rituximab does not seem to have a significant effect on serum immunoglobulin levels, but its administration could be associated with diminution of autoantibody levels.17,18
There are different preliminary studies on the therapy of SLE and other autoimmune diseases with Rituximab.19–24 In this regard, it has been reported that this biological agent seems to induce clinical improvement and diminution of the abnormalities found in B lymphocytes in patients with this condition.25–28 In addition, we have previously found that the addition of Rituximab to the immunosuppressive therapy of patients with refractory lupus nephritis resulted in a marked improvement in both, renal function and disease activity.29 Furthermore, it has been reported that Rituximab therapy of SLE patients is associated with diminution of activation markers of T lymphocytes, including the expression of CD40L, as well as with a significant effect on the levels and function of T regulatory cells, and the induction of T cell apoptosis.29,30
In an open clinical trial we have explored the possible therapeutic effect of Rituximab in SLE patients with severe manifestations, specifically with severe nephritis or CNS affectation or massive pulmonary hemorrhage. Our data strongly suggest that Rituximab exerts an important beneficial effect in most of these patients. In our previous report we emphasize that patients with severe nephritis (type IV: 18, type III and type V: 2) had improvement of levels and function of T regulatory cells and induction of T cell apoptosis, immunological facts which could explain the clinical response to diverse dosages of rituximab in spite do not have increased steroids therapy or do not have used steroids. In this paper we show our clinical data of this group of patients, besides two additional groups, one of them with very serious neurological affectation and the other one, three patients with massive pulmonary hemorrhage.
Patients and methodsThirty-one SLE patients with severe disease, and in most of them refractory to conventional intensive treatment were enrolled in a preliminary open and prospective clinical trial. Patients had severe lupus manifestations, twenty-two with lupus nephritis, six with neuropsychiatry manifestations, and three with massive pulmonary hemorrhage. All patients fulfilled the classification criteria of the American College of Rheumatology for the diagnosis of SLE,31 and disease activity was scored according to the MEX-SLEDAI index.32 Most of our patients with lupus nephritis had WHO type IV (18), and less frequent were type II (2) and V (2); all of them under DMARD's therapy and most with variable doses of steroids, with persistent activity, through proteinuria, urinary red or white cells or casts. Following was made with creatinine levels, diary proteinuria and creatinine clearance. Patients received Rituximab 500 to 1000 mg once or twice, at 2 week intervals, and the therapeutic response was evaluated by clinical and laboratory parameters. The rituximab dosage varied because economic limitation; previously we have used low dosage of rituximab in diverse autoimmune diseases (rheumatoid arthritis, dermatomyositis, and systemic lupus erythematosus) with similar results (B cell depletion, immune-regulatory effects, and clinical response) of those reported with conventional approved doses. All patients were pretreated with dexametasone (8 mg i.v.) or prednisone, (40 mg., p.o.), and loratadine (10 mg p.o.). Infusion of Rituximab started slowly (15 drops/min), increasing each 20 min. Because patients with renal involvement were under immunosuppressive therapy (most of them with 2 or more DMARD's) when occurred their renal exacerbation or relapse, we decided that did not necessary to increase steroid dosage and maintain without this drug in 6 of them. We included here, two other patient groups, one with severe central neurologic involvement and the other one with acute massive pulmonary hemorrhage, and all of these patients received high doses of metylprednisolone together. Massive pulmonary hemorrhage was defined characteristically with severe acute respiratory failure with diminution of 3 or more grams of haemoglobin without any other potential responsible causes; so, three patients with massive pulmonary hemorrhage diagnosis were treated with 1 g of rituximab, and additionally received methylprednisolone (at least 1 g/day for 4 days), cyclophosphamide (500 mg/m2), and azathioprine (150 mg/day) or methotrexate (15 mg), beside diary hemodialysis. When data of an allergic reaction were detected, infusion was stopped, and hydrocortisone was administered (100 mg i.v.). In these cases, after a careful clinical evaluation, Rituximab infusion was re-started, upon the continuous monitoring of these patients.
To evaluate differences from basal and 60 days post-rituximab therapy, we used decriptive statistical analysis and comparison media values with t student.
ResultsWe have previously reported that the addition of Rituximab to the immunosuppressive therapy of patients with refractory lupus nephritis is associated with significant improvement in different clinical and laboratory parameters.29 All, except two of them had B cell depletion with 500 to 2 g of rituximab. All patients were under intensive immune-regulatory therapy and we decided that in spite renal activity the dose of glucocorticoids remained unchanged during the study, and in six cases whose did not are with steroid therapy, these drugs were not administered, but the patients continued with their DMARD's therapy.
In the Table 1, we show the demographic characteristics of our patients with SLE and severe glomerulonephritis. As previously reported in these patients, Rituximab therapy induced a significant improvement in different clinical parameters in most of them, in spite the administration of only 1.0 g of this biologic agent. It is worth mentioning that in six of these patients, no glucocorticoids additionally to those associated to rituximab infusion were administered and that in the rest of the group the dose of these anti-inflammatory drugs remained unchanged. In these patients with severe glomerulonephritis, the disease activity (MEX-SLEDAI index) significantly diminished (p<0.05) as well as proteinuria, in most of them (from 3.710 g/L to 1.786 g/L, p<0.05). Diminution of proteinuria occurred as early as at day 8 after the first infusion of Rituximab. Creatinine clearance increased in 72% of patients, but this variation did not reach statistical significance (73 to 86 ml/1.73 m2), neither the apparent diminution of serum creatinine levels (0.97 to 0.78 mg/dl).
Table 1. Demographic characteristics of SLE patients included in the study
| Age/gender | Time of evolution | Clinical manifestations | Nephritis type/duration (years) | Disease activity * | Previous therapy |
| 41/F | 15 | OU, Ar, Le, Ly | IV/8 | 12 | GC, Cyc, Aza |
| 24/M | 4 | Er, Ph, Le | IV/4 | 10 | GC, Aza, Mmf, CsA |
| 36/F | 6 | Er, Ph, OU, Ar, Le, Ly | III/6 | 14 | GC, Aza, CsA |
| 19/M | 5 | Le | IV/4 | 8 | GC, Cyc, Mmf |
| 56/F | 12 | Er, Ph, OU, Se, Ar | IV/3 | 15 | GC, Cyc, Mmf, Aza |
| 9/M | 1 | Er, Ph, Se, Ar | IV/1 | 12 | GC, Mmf, Mtx, Aza, Cyc |
| 26/F | 11 | Er, Ph, OU, Se, Ar, Le | IV/4 | 10 | GC, Mtx, Aza |
| 31/F | 16 | Ph, OU, Se, Ar, CNS | IV/8 | 10 | GC, Cyc, Lfm, Aza, Mtx, Mmf |
| 23/F | 3 | Ph, OU, Se, Ar, CNS | IV/3 | 12 | GC, Mmf, Aza, Cyc, Mtx |
| 32/F | 6 | Er, Ph, OU, Ar, Ly | IV/6 | 8 | GC, Mtx, Aza, Cyc |
| 24/F | 2 | Er, Ph, OU, Se | III/2 | 9 | GC, Mtx, Aza |
| 27/F | 13 | Er, Ph, OU, Se, Ly | IV/13 | 19 | GC, Mmf, Cyc, Aza |
| 43/F | 6 | Er, Ph, Le | IV/6 | 9 | GC, Mtx, Aza, Pdn |
| 21/F | 8 | Er, Ph, OU, Se, Ly | V/8 | 9 | GC, Mtx, Aza, Cyc, Mmf |
| 36/F | 1 | Er, Ph, Ly | V/1 | 10 | GC, Aza |
| 25/F | 1 | Er, OU, Se, Ly, CNS | IV/1 | 11 | GC, Aza, Mtx |
| 33/F | 8 | Er, Ph, OU, Se, Ar, Le | IV/8 | 10 | GC,Aza, Mtx, Mmf, Cq |
| 28/F | 3 | Er, Ph, OU, Se, Le, Ly | IV/3 | 9 | GC, Cyc, Aza, Mmf, Mtx, Cq |
| 40/F | 4 | Er, Ph, OU, Se, Ar | IV/4 | 10 | GC, Aza, Mtx |
| 19/F | 1 | A, Le, Ly | IV/1 | 10 | GC, Aza, Mtx, Mmf |
| 24/F | 4 | Er, Ph, Le | IV/3 | 10 | GC, Aza, Mtx, Mmf, Cyc |
| 22/F | 1 | OU, Ar, Le, Se | IV/1 | 10 | GC, Aza, Mtx, Mmf, Cq |
Ar: arthritis; Aza: azathioprine; CNS: central nervous system; Cq: chloroquine; Cyc: ciclosporine; Er: erythema; GC: glucocorticoids; Le: leukopenia; Lfm: leflunomide; Ly: lymphopenia; Mmf: mycophenolate; Mtx: methotrexate; N: nephritis; OU: oral ulcers; Ph: photosensitivity; Se: serositis; SLE: systemic lupus erythematosus.
* MEX-SLEDAI index.
Five of our patients showed a complete remission of renal disease (defined as normal renal function, inactive sediment and proteinuria <500 mg/L), and seven patients showed a partial renal response (stable renal function, inactive sediment and diminution of proteinuria >50%). Six additional patients exhibited improvement in one or several renal parameters, however the other two patients did not show improvement of any renal function parameter and had renal failure at days 60 and 90. We observed that 9/12 patients with nephrotic proteinuria had diminution >50% and in 3, the proteinuria disappeared. Long-term follow-up of these patients (2.0 years) showed that five of them relapsed. After an additional administration of 1.0 g of Rituximab, in three of them a good clinical and laboratory therapeutic response was observed.
As we mentioned previously, most of patients with severe glomerulonephritis (20/22) that received Rituximab had evidence of B cell depletion and only one of them had a severe and fatal infection with massive pulmonary haemorrhage associated to pulmonary histoplasmosis and arterial coronary mucormycosis. This patient was under mofetil mycophenolate therapy, and had an episode of diabetic ketoacidosis.
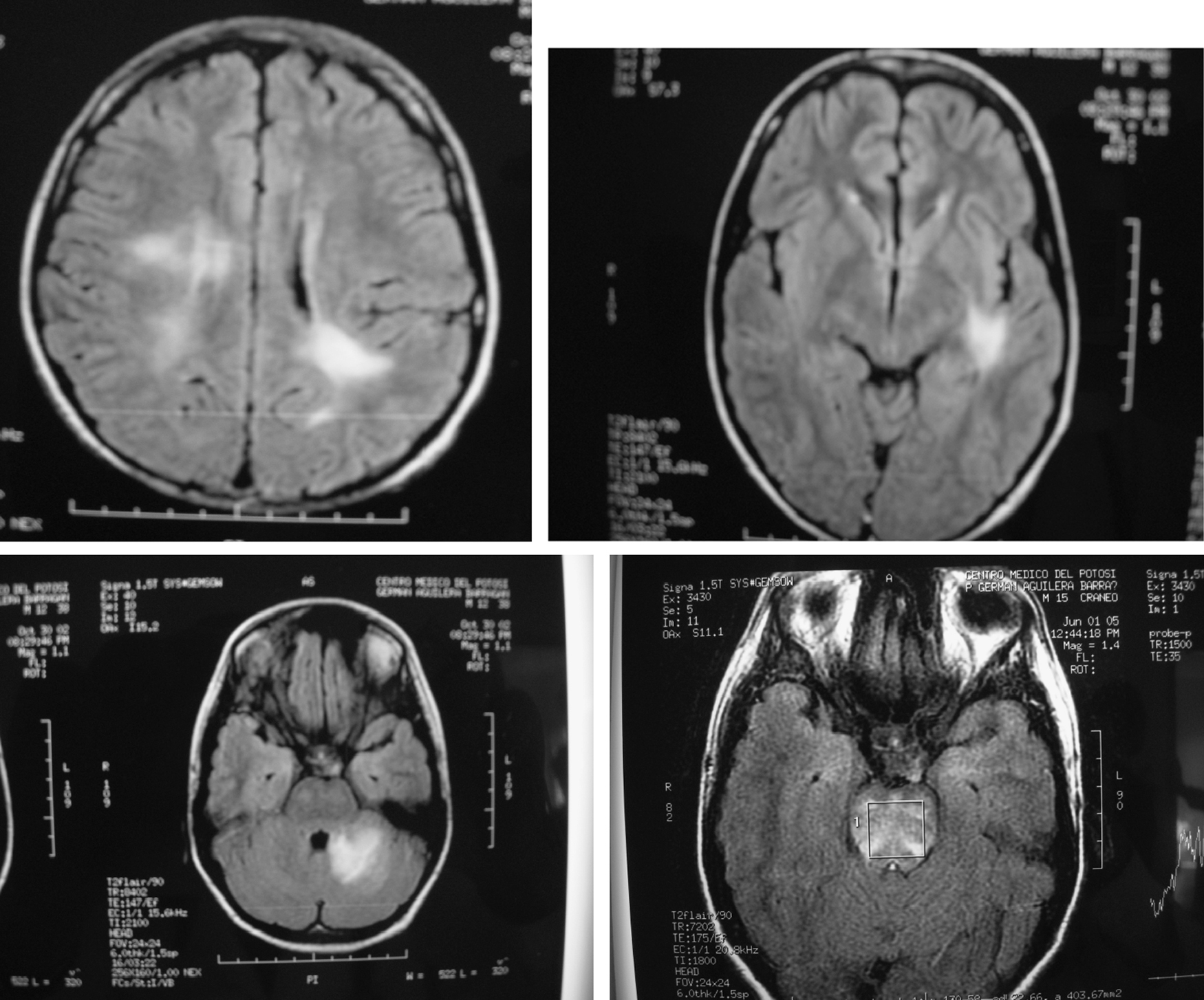
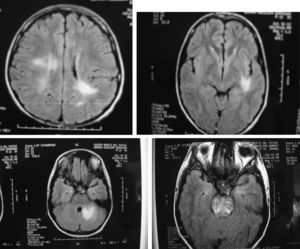
In our group of six SLE patients with severe central nervous system (Table 2) disease we observed an excellent response to Rituximab therapy. In all cases no permanent damage or disability was observed, including in the two patients with transverse myelitis, one with meningo-encephalitis, one with cerebellar syndrome, one with hemorrhagic stroke and one with severe choreoathetotic movements. Patients with transverse myelitis received rituximab in their second (case 1) and third (case 2) episodes, and in this last episode of the second patient who had sensitive level and distal dysesthesias, we decided do not use methylprednisolone pulses (mPDN), and in both patients, in their relapses, we did not use cyclophosphamide; both of them, had complete resolution of this severe neurologic involvement. We realized MRI studies only in their 1st myelitis presentation, and in both cases, were normal. Patient 4, had MRI totally normal, in fact she was well until 3 months after her had received rituximab 1 g, when she presented again a cerebellar affectation with ataxia and a new MRI study was also normal; she was treated with rituximab 1 g and mPDN pulses with complete resolution 15 days after and she continue healthy. The other 3 patients have had MRI abnormalities; in Fig. 1, we show some of images of MR related to patient 3, who achieve complete clinical remission and IRM normal, study that was taken 2 months after were treated with rituximab 1g; he is healthy 3 years after and is being treated with hidroxi-chloroquine and simvastatine.
Fig. 1. RMI with various hyperintensive lesions which disappear after therapy with rituximab (Patient described above as case 4).
Table 2. Characteristics of SLE patients with severe neurologic manifestations included in the study
| Diagnosis/Evolution | Evolution | Clinical manifestations | CNS involvement | Treatment |
| Female, 18 | 4 | E, Ph, OU, serositis, Ar | TM | CPM, mPDN |
| TM, Op N | + Rituximab 1g | |||
| Female, 43 | 14 | E, Ph, OU, serositis, Ar | CPM, T TM, MM | CPM, mPDN, |
| Pulmonary hypertension, distal/ | TM, MM | Mtx, statins | ||
| esophageal sclerosis | TM, MM | Rituximab 2g | ||
| Male, 15 | 2 | Intense headache, fever | M-Encephalitis | mPDN, statins |
| Low creatinine clearance | Rituximab 1g | |||
| SLE and APL nephropathy | ||||
| Female, 15 | 0 | Cerebellous syndrome, | Cerebellitis | mPDN, CPM, |
| Polyarthritis, pericarditis, | Psycosis | statins, | ||
| Auditory allucinations | Rituximab 1g | |||
| Female, 25 | 0 | Chorea, arthritis, psicosis | Choreo-athetosis | mPDN, statins |
| movements, Psicosis | R Rituximab 0.5g | |||
| Female, 31 | 11 | E, Ph, OU, serositis, Ar, | Hemorrhagic stroke | Dexa, CPM, statins |
CNS: central nervous system; E: erythema; Ph: photosensitivity,; OU: oral ulcers; Ar: arthritis; TM: transverse myelitis; Op N: Optical neuritis, CPM: cyclophosphamide; mPDN: methylprednisolone; MM: multiple mononeuritis; Mtx: methotrexate; APL: anti-phospholipid; Dexa: dexametasone.
The three female patients with massive pulmonary hemorrhage that received Rituximab had a fatal course despite the concomitant administration of mPDN pulses (average 6.0 g each), combined with cytotoxic drugs and at least 3 hemodialysis sessions. Patients received one cycle of cyclophosphamide 500 mg/m2. Probably in these cases, rituximab was not a good therapy because patients died in a few days after they started with their clinical manifestations of acute severe respiratory failure. All patients had renal, dermatologic and rheumatologic affectations, besides to have positive antinuclear antibodies and two of them hypocomplementemia.
Clinical examples- 1. A nine years old boy, with asymmetric oligoarthritis, a papular skin rash, abdominal pain, and evidence of renal disease was admitted to the emergency room. Schönlein-Henoch syndrome diagnosis was established, and prednisone 0.5 mg/kg/day improved his clinical manifestations. However, a renal biopsy showed diffuse and generalized glomerulonephritis, with crescents in 3/15 glomerulus, and immune granular deposits of IgG, IgA, IgM, C3 and C1q. An aggressive immunosuppressive therapy was started with prednisone 1.0 mg/kg/day, cyclophosphamide, azathioprine and cyclosporine, but no favorable response was observed with an enhanced proteinuria (1.25 g/L), heavy erythrocyturia, numerous casts, and a significant diminution in creatinine clearance (60 ml/min). Rituximab (0.5 g, two infusions, at days 1 and 15) was added to his therapy and a significant improvement in most clinical (asymptomatic) and laboratory (proteinuria of 425 mg/ L, creatinina clearance of 85 ml/min) parameters was observed. However, an active urinary sediment persists 18 months after Rituximab therapy, and actually his therapy is with mycophenolate, low doses of steroids, methotrexate, statins, angiotensin inhibitors and vitamin D. The interest to report this patient is because Schönlein-Henoch purpura is a rare SLE presentation, but in spite adequate therapy persist with glomerulonephritis activity.
- 2. A 21 years old female with diagnosis of SLE, and under cytotoxic therapy because type IV lupus nephritis was admitted because photosensitivity, facial erythema, polyarthralgias, myalgias, transitory pleuritic chest pain, and fever, with an active urinary sediment and 3.25 g/L of proteinuria. One week later of rituximab therapy, proteinuria fall to 1.75 g/L, and no casts or erythrocytes were detected in a new urine examination. Two months later, most clinical manifestations significantly improved and proteinuria further fall to 300 mg/L, without any evidence of sediment active or renal activity. Long-term follow-up (2.0 years) showed that she is asymptomatic with low levels of proteinuria. This patient is an example of a good and prolonged response to depletory B cell therapy; besides we recognized that in most of literature reports as in our group of patients anti-CD20 therapy, the response is expressed generally after 4 or more weeks. Patient came with us 8 days after with a new urine test then of various with more than 3 grams of proteinuria previously to biologic therapy mentioned.
- 3. A 26 years old female with SLE and a previous episode of disseminated histoplasmosis (five years ago) was admitted with photosensitivity, facial erythema, oral ulcers, pleuritic chest pain, fever, general malaise, tachycardia, thrombocytopenic purpura and proteinuria of 1.8 g/L. Rituximab, 1.0 g in two doses, was added to the immunosuppressive therapy, and one-month later most clinical manifestations had disappeared. Nineteen months later she is asymptomatic, with normal platelet counts and no proteinuria.
- 4. A 15 years old male with SLE and a history of encephalitis and meningitis that was treated with prednisone and complete antituberculous therapy (9 months). He had been well, but he was admitted because severe headache with an MRI showing new brain lesions (Fig. 1), and manifestations of renal damage with a diminished creatinine clearance (70 ml/min). Methylprednisolone pulses (1 g/day for 3 days), besides 2 g of Rituximab divided in two doses was prescribed. One month later most clinical manifestations improved, and five months later, the lesions seen in the MRI disappeared. At this time, a renal biopsy showed mild endothelial thickness without glomerulonephritis, with scarce granular deposits of IgG, IgM, IgA and C3. Three years after Rituximab therapy the patient remains asymptomatic, with creatinine clearance of 123 ml/min, a normal urine test, with no glucocorticoids and receiving only low doses of atorvastatine and hydroxychloroquine.
Patients with SLE and severe multiorganic affectation represent a therapeutic challenge. Although we have now more efficient immunosuppressive drugs, their unspecific effect is largely associated to severe side effects. In addition, in some cases this type of nonspecific and aggressive immunosuppressive therapy is not sufficient to stop disease activity. Accordingly, a more specific immunosuppressive therapy for the treatment of different inflammatory autoimmune diseases is required, an aim that is significantly facilitated by our current knowledge of the pathogenesis of SLE. In this regard, it is evident the important role of B lymphocytes in SLE, through de synthesis of different cytokines, their involvement as antigen presenting cells, and as the source of the different auto-antibodies seen in this condition. Therefore, B lymphocytes appear to be a good specific target for novel therapies in SLE.
Rituximab is a chimerical monoclonal antibody directed against CD20, a molecule that is specifically expressed by B lymphocytes. It has been found that the administration of this antibody exerts interesting expected and unexpected effects. Thus, the long-term depletion of B cells is a well-documented effect of Rituximab administration.33 In addition, we have previously reported the enhancement of regulatory T cells by Rituximab as well as the induction of T cell apoptosis by this biological agent.29
Our findings further support the previous reports on the beneficial effect of Rituxumab administration in patients with SLE, mainly in patients with severe and refractory nephropathy as well as those with CNS disease.23,34–36 However, this biological agent does not seem to have a role in the therapy of the massive pulmonary hemorrhage associated to SLE, at least for the treatment of the acute, severe manifestations of this condition, potentially fatal in a few days, episodes of short time that may be do not permit the action of immune therapy including rituximab.
Most of our patients with severe lupus nephropathy had a dramatic improvement after Rituximab administration. However, two patients showed progression to renal failure, one of them died and the other one is in hemodyalisis. One of these patients had serious mixed acidosis conditioned by uncontrolled diabetes mellitus and an undetected infectious process. At necropsy, two fungal infections were detected, histoplasmosis in the lungs, and mucormycosis in a coronary artery. However, according to our study, Rituximab, although is a potent inducer of B cell depletion, and show a strong anti-inflammatory/immunosuppressive effect, its administration seems to be rarely associated to serious adverse events, even when is combined with different immunosuppressive drugs. In addition, Rituximab seems to maintain its therapeutic effect for months or even years, as we describe here, and it has been reported by others, but frequently because relapses in the cases of severe lupus nephritis we should consider a new course of this B cell depletory therapy. 23–30,37
We consider that it would be of interest to assess the efficacy of Rituximab as a single immunosuppressive agent for the therapy of severe SLE. We think that it is possible that, at least in some cases, Rituximab alone would be able to exert a significant beneficial effect, and even, in some patients, to induce disease remission. In this regard, it is worth mentioning that in six patients of our study, the beneficial effect of Rituximab was exerted without the concomitant administration of glucocorticoids. In this regard, these data suggest that glucocorticoids could be not as important in Rituximab therapy of autoimmune diseases as previously thought, in particular when patients are being treated with immune-regulatory agents, and additionally if we do not increase or prescribe steroids, we will have less immunosuppressive effects associated to this therapy, but with similar efficacy. Finally, it is important to keep in mind that we performed an open preliminary trial and that our results on the apparent beneficial effect of Rituximab in severe SLE should be validated through a large controlled and blinded study.
Autor para correspondencia.
Carlos Abud-Mendoza
Dirección: c_abud@hotmail.com