Embolic and constitutional manifestations of intracavitary cardiac tumors are included within the classic mimickers of systemic vasculitis, especially in those in which there are no cardiac manifestations. We present a case report of atrial myxoma in which the patient only presented systemic symptoms and in whom an initial diagnostic approach of systemic vasculitis was made. We also performed a literature search of the cases described.
Patient and methodA case report of atrial myxoma with atypical presentation manifested as a systemic disease with no concomitant cardiac symptoms is described. The case report is discussed and 11 cases of atrial myxoma pseudovasculitis described in the literature are reviewed, emphasizing their similarities and differences.
DiscussionConstitutional symptoms and cutaneous manifestations were the most common. Most of the cases showed partial response to glucococorticosteroid treatment, reinforcing the theory of the inflammatory role in its pathogenesis. Mean delayed time to diagnosis was 12.27 months.
ConclusionAtrial myxoma is a systemic vasculitis mimicker, this being difficult to diagnose in the absence of cardiac manifestations. This delay in diagnosis entails serious complications.
Las manifestaciones embolígenas y constitucionales de los tumores cardíacos intracavitarios se engloban dentro de los mimetizadores clásicos de las vasculitis sistémicas, sobre todo en aquellas ocasiones donde no se presentan manifestaciones cardiológicas. Se describe un caso de mixoma auricular con clínica exclusivamente sistémica, cuya orientación diagnóstica inicial fue de vasculitis. Se revisan los casos descritos en la literatura.
Paciente y métodoSe describe un caso de mixoma auricular con presentación en forma de manifestaciones sistémicas sin sintomatología cardiológica acompañante. Se expone el caso clínico y se compara con 11 casos de seudovasculitis por mixoma auricular descritos en la literatura, haciendo énfasis en las similitudes y divergencias.
DiscusiónLos síntomas constitucionales junto con las manifestaciones cutáneas fueron los más frecuentes. La mayoría de los casos presentaban respuesta parcial al tratamiento glucocorticoideo, reforzando la teoría del componente inflamatorio en su patogenia. La demora media en el diagnóstico fue de 12,27 meses.
ConclusiónEl mixoma auricular es un simulador de vasculitis sistémica y es de difícil diagnóstico cuando no presenta manifestaciones cardíacas. La demora diagnóstica puede conllevar complicaciones graves.
The systemic manifestations of intracavitary cardiac tumors—fever, weight loss, joint and muscle pain, and Raynaud's phenomenon—lead to this entity's being mistaken for immunological, neoplastic or infectious diseases, especially in those cases in which there are no cardiac manifestations.1 There is a large group of classical mimickers of systemic vasculitis, including atrial myxoma, atherosclerosis, systemic AL amyloidosis, bacterial endocarditis, antiphospholipid syndrome and thromboangiitis obliterans.2 This masking phenomenon is well known, although there have been few reports of cases in which the cause was atrial myxoma. We present a case of atrial myxoma with embolic and systemic manifestations, but no symptoms or signs of cardiac involvement, in which the initial findings pointed to a diagnosis of systemic vasculitis. We report the details of a search for the cases described in the literature, analyzing similarities and differences.
Clinical ObservationThe patient was a 60-year-old man with a history of hypertension, which is being treated with an angiotensin receptor blocker, and dyslipidemia, which is being treated with a statin. He presented with low back pain of acute onset, as well as acrocyanosis of the right hand associated with ischemic pain in the fingers of that hand. Physical examination revealed acrocyanosis of the right hand; the peripheral pulses were present and symmetric. The heart and lung sounds were normal. He underwent chest radiography, electrocardiography and computed tomographic angiography of thorax and abdomen, all of which were normal. Laboratory tests, including complete blood count, kidney and liver function, coagulation tests, antiphospholipid antibodies, cryoglobulins and serological tests for hepatitis B and C viruses, parvovirus and antineutrophil cytoplasmic antibodies (ANCA) were normal. Antinuclear antibodies were detected at low titers (1/80) in a homogeneous pattern, with negative anti-double-stranded DNA antibodies and no complement consumption. Nailfold capillaroscopy revealed signs of angiogenesis with internal bleeding in the fingers of the right hand. Treatment consisted of oral antiplatelet therapy and intravenous prostaglandins, which achieved the complete resolution of the symptoms. Three months later, the patient developed bilateral Raynaud's phenomenon. One month after that, he presented with acute, stabbing, epigastric pain, as well as ischemic pain in the 4th toe on his left foot, hypesthesia in the 2nd–4th toes on the same foot and pain in the plantar fascia upon stepping with that foot. He reported proximal muscle weakness in lower extremities and undetermined weight loss over the preceding 2–3 months. Physical examination revealed that his left foot was colder than his right foot, a mild erythematous lesion in left plantar fascia, bilateral absence of posterior tibial and pedal pulses, and palpable popliteal pulses. The first heart sound had a greater intensity and there were no extra sounds.
The results of the laboratory tests included a hemoglobin level of 10.4g/dL, hematocrit 34%, mean corpuscular volume 78fL, creatine kinase 602U/L, creatine kinase-mb 26U/L, platelets 506,000/mm3, γ-glutamyl transpeptidase 216U/L, alkaline phosphatase 200U/L, C-reactive protein 128.1mg/L and erythrocyte sedimentation rate 88mm/h. The remaining laboratory findings were normal. As we suspected medium to small vessel systemic vasculitis, the patient was admitted to the hospital to complete the study.
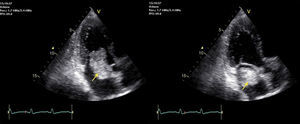
An electromyogram revealed mononeuritis of left posterior tibial nerve with no evidence of myopathy. To rule out arteriographic abnormalities, contrast-enhanced computed tomographic angiography of thorax and abdomen was scheduled. The patient received glucocorticoids (60mg of methylprednisolone in 2 doses) to prevent a probable allergic reaction to the contrast material, which resulted in a notable improvement in his symptoms. The imaging study revealed an intracavitary filling defect at the level of left atrium. This finding led to the performance of transthoracic echocardiography, which disclosed the presence of a large pedunculated tumor in left atrium that was attached to the atrial septum at the level of the fossa ovalis, which prolapsed toward the valve in diastole and was associated with mild mitral regurgitation (Fig. 1).
In view of the findings, it was decided that the patient be transferred to another center for surgery to excise the atrial mass, which was performed with cardiopulmonary bypass. During the immediate postoperative period, his course was favorable, although he developed an ischemic skin lesion on the pad of the first toe of his left foot. The pathological study of the surgical specimen (6.5cm×5cm×1.5cm) confirmed the diagnosis of atrial myxoma.
The review of the literature was carried out using the PubMed search engine with the search terms “pseudovasculitis”, “cardiac tumor” and “atrial myxoma”,3–13 and limiting the search to texts in English, Spanish, French or German. As a result, we found 11 case reports spanning a period of 36 years (1978–2014).
The clinical characteristics of the cases reviewed are shown in Table 1. The patients included 8 men and 3 women, with a mean age at diagnosis of 39.73 years (range, 17–88).
Clinical Manifestations in the Cases of Cardiac Tumors Mimicking Vasculitides or Connective Tissue Diseases Reported in the Medical Literature.
| Clinical manifestations | Case no. | Mano et al. (2014)3 | Hartig et al. (2014)4 | Patel et al. (2009)5 | Nishio et al. (2005)6 | Rivero et al. (1998)7 | Gravallese et al. (1995)8 | Bodokh et al. (1993)9 | Boussen et al. (1991)10 | Thomas et al. (1981)11 | Byrd et al. (1980)12 | Huston et al. (1978)13 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic | |||||||||||||
| Weakness | • | • | • | • | • | • | 9/12 | ||||||
| Fever | • | • | • | • | • | ||||||||
| Weight loss | • | • | |||||||||||
| Cutaneous joint and muscle pain | • | • | • | • | • | • | • | • | 8/12 | ||||
| Erythema | • | • | • | • | • | • | • | • | 9/12 | ||||
| Purpura | • | • | • | • | |||||||||
| Acrocyanosis | • | • | • | • | 4/12 | ||||||||
| Raynaud's phenomenon | • | • | • | 3/12 | |||||||||
| Ischemic pain | |||||||||||||
| Body parts forming a tip, e.g., tips of fingers and toes | • | • | • | • | • | • | 8/12 | ||||||
| Calf | • | • | • | • | |||||||||
| Mono/polyneuritis | • | • | • | 3/12 | |||||||||
| Neurological | |||||||||||||
| Headache | • | • | • | 7/12 | |||||||||
| Stroke | • | • | • | • | • | ||||||||
| Amaurosis | • | • | |||||||||||
| Seizures | • | ||||||||||||
| Central vertigo | • | • | |||||||||||
| ACS | • | • | 2/12 | ||||||||||
| Absent peripheral pulses | • | 1/12 | |||||||||||
| Heart sounds | |||||||||||||
| Murmur | • | • | • | 5/12 | |||||||||
| Loud S1 | • | • | |||||||||||
| Improvement with glucocorticoids and/or IT | • | • | • | • | • | • | • | • | • | • | • | 11/12 | |
| Death | • | • | 2/12 | ||||||||||
ACS, acute coronary syndrome; IT, immunosuppressive therapy; S1, first heart sound.
In 8 of the published cases, the authors reported an increase in acute phase reactants, with a mean erythrocyte sedimentation rate of 58.37mm/h (range, 30–108). In addition to the increase in acute phase reactants, anemia was identified as another possible cause of alterations in laboratory findings in atrial myxoma.14 This condition was also observed in our patient, although it was only mentioned in 2 of the reviewed texts. Immunological markers were negative, except in 1 patient with a positive test for ANCA in a perinuclear pattern, another who tested positive for anti-double stranded DNA antibodies and complement consumption, and a third with circulating lupus anticoagulant.
As could be expected, this atypical presentation resulted in a diagnostic delay in every case,3–13 with an average of 12.37 months (range, 1–36) between symptom onset and the final diagnosis.
All the patients were ultimately diagnosed with atrial myxoma after a histological study. With regard to the size of the tumor (reported in 5 cases), the largest12 measured 8cm×4.5cm×4cm and the smallest,5 2.5cm×1.8cm×1.4cm. All of them were unifocal, with the exception of 1 case that was described as multifocal atrial myxoma. That patient also had an acute cardiac event and an indolent course that ultimately led to his death.10
DiscussionThe myxoma is the most common benign primary cardiac tumor, accounting for up to 50% of all such lesions. It is most frequently located in left atrium (75%), mainly on the interatrial septum, bordering on the foramen ovale.1 The clinical presentation can be classified according to 3 forms: (a) cardiac symptoms of mitral or tricuspid regurgitation secondary to intracavitary obstruction, depending on the location of the myxoma, which is found in up to 67% of the cases; (b) embolic events involving myxomatous material (29%), in the form of ischemic skin lesions and mononeuritis due to involvement of the vasa nervorum, with stroke being the prevalent event; and (c) systemic symptoms such as fever, weight loss, joint and muscle pain, vasospastic attacks and cutaneous purpura, all clinical forms that can coexist. There can be abnormal laboratory values, such as anemia, increase in acute phase reactants, hypocomplementemia and, occasionally, positivity for immunological markers. The laboratory values usually return to normal after resection of the tumor.1,14
The clinical presentation depends partly on the size, the site and the mobility of the tumor, with left atrium being the most widely reported location.15 The systemic symptoms have been related to the interleukin 6 concentration.16 The fact that a chain of cytokine activation plays a role in the pathophysiological mechanism, together with the clinical response to glucocorticoid and immunosuppressive therapy, supports the hypothesis proposed by some authors of a possible vasculitic nature.16,17
When the clinical presentation involves only embolic events and systemic symptoms, the diagnosis is delayed. In all of the cases reviewed here, the diagnosis was based on transthoracic echocardiography, which had been requested for different reasons in accordance with the diagnostic process, for example, to complete a study following a stroke.6,7,10,12,13
ConclusionAtrial myxoma is a mimicker of systemic vasculitis, and is difficult to diagnose when there are no cardiac manifestations. The diagnostic delay can result in serious complications involving embolic events, one of the most feared of which is stroke.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Moreno-Ariño M, Ortiz-Santamaria V, Deudero Infante A, Ayats Delgado M, Novell Teixidó F. Un simulador clásico de vasculitis sistémica. Reumatol Clin. 2016;12:103–106.