The current guidelines in the treatment of rheumatoid arthritis (RA) include the early diagnosis and early use of disease modifying drugs to achieve remission or low disease activity level, known as “Treat to Target” (T2T). The objective of this study is to develop a composite indicator (CI) to evaluate the quality of care in the management of patients with RA, according to the T2T strategy and other general recommendations concerning the management of these patients.
Material and methodThe phases of the construction of the CI were: (1) selection of quality criteria through expert judgement; (2) prioritisation of the criteria, according to relevance and feasibility, applying the Delphi methodology (two rounds) involving 20 experts; (3) design of quality indicators; and (4) calculation of the weighted CI, using the mean value in relevance and feasibility granted by the experts. The source of information for the calculation of the CI are the medical records of patients with RA.
ResultsTwelve criteria out of 37 required a second Delphi round. Thirty-one criteria were prioritised. These criteria presented a median in relevance and feasibility greater than or equal to 7.5, with an interquartile range of less than 3.5, and a level of agreement (score greater than or equal to 8) greater than or equal to 80%.
ConclusionsThe constructed CI allows us to evaluate the quality of care of patients with RA following the T2T strategy in the rheumatology units of Spanish hospitals, offering a valid and easily interpretable summary measure.
El paradigma actual en el tratamiento de la artritis reumatoide (AR) contempla el diagnóstico temprano y el uso precoz de fármacos modificadores de enfermedad (FAME) para alcanzar la remisión o baja actividad inflamatoria, lo cual, se conoce como «treat to target» (T2T). El objetivo del trabajo es desarrollar un indicador compuesto (IC) para evaluar la calidad asistencial en el manejo de los pacientes con AR atendiendo a la estrategia T2T y a otras recomendaciones generales para la atención de estos pacientes.
Material y métodoLa construcción del IC siguió las fases: 1) selección de los criterios de calidad mediante un juicio de expertos; 2) priorización de los criterios, a partir de un Delphi con 20 expertos; 3) diseño de los indicadores de calidad, y 4) cálculo del IC ponderado. La fuente de información para el cálculo del IC son las historias clínicas de los pacientes con AR.
ResultadosDe los 37 criterios seleccionados, 12 necesitaron una segunda ronda Delphi. Se priorizaron 31 criterios, los cuales presentaron una mediana en relevancia y factibilidad, en las rondas Delphi, mayor o igual a 7,5, con un rango intercuartílico inferior a 3,5, y un grado de acuerdo (puntuación mayor o igual a 8) igual o superior al 80%.
ConclusionesEl IC construido, consensuado y ponderado, permite evaluar la calidad asistencial de los pacientes con AR, en las Unidades de Reumatología de hospitales españoles, ofreciendo una medida resumen válida y fácilmente interpretable.
The strategic objectives of the Spanish Rheumatology Society include promoting care quality in daily clinical practice, establishing quality criteria and standards for the care of patients with rheumatological diseases and ensuring their follow-up, updating and monitoring, thus completing a cyclical process of continuous quality improvement.
Among the wide range of rheumatic diseases, rheumatoid arthritis (RA) has undergone a revolution over recent decades because of the major importance of early diagnosis and prompt treatment with disease-modifying drugs (DMDs), which are essential to control the activity of the disease in the medium and long term, and modify its prognosis.1–4 Other factors that have contributed to this therapeutic paradigm shift are a stricter and more precise evaluation of the activity of the disease, the application of the recommendations for the appropriate use of methotrexate (MTX) in combination with folic or folinic acid, recognised as a potent drug for controlling the disease, and from the year 2000, the introduction of biological therapy. This new knowledge has become consolidated over recent years, and in 2010 a set of international recommendations were published for the strict control of the treatment of RA in daily clinical practice, known as “treat to target” (T2T).5 These were updated in 2016,6 setting out as key pillars for the clinical management of these patients the definition of treating to target, understood as periodically evaluating the inflammatory activity by composite activity indices until remission or low inflammation activity is achieved, as well as a dynamic interrelationship between the empowered patient and the rheumatologist, where therapeutic adjustments are made at each visit as the targets set are achieved.
All of the above, along with the implementation of general recommendations on the clinical care of RA patients, will contribute towards improving the quality of care for these patients. It is in this context that the ARExcellence (assessment of the care quality in the clinical management of patients with RA in rheumatology outpatient clinics) project was born, which uses a composite indicator (CI) methodology to develop a standardised process of evaluation and monitoring of care quality including criteria from the T2T strategy, from general recommendations on prevention and health promotion measures (healthy life habits, immunisation and control of cardiovascular risk), from the pharmacological management of conventional DMDs and glucocorticoids, and from the organisation of rheumatology units (RU). The aim of this paper was to construct a CI, as a summary measure of care quality in the clinical management of RA patients attended in the rheumatology departments of Spanish hospitals.
Material and MethodsGroup of ExpertsIn order to construct the CI a group of experts was formed that comprised the principal investigator (PI), the scientific committee (SC) of the ARExcellence, and 16 panellists. The SC comprised 3 rheumatologists, 2 of them heads of care level 5 hospital departments (very large hospitals, of great structural weight and a lot of care activity; over 900 beds; very technologically advanced and with a complex service portfolio), with wide experience in the clinical management of patients with RA, in care quality and epidemiological research. The third member of the project's SC and the PI are attending rheumatologists from level 5 hospitals, who also have wide experience in the clinical management of patients with RA, in care quality and epidemiological research. The 16 panel members were chosen based on their experience in the clinical management of patients with RA and their knowledge of quality of care. Efforts were made to ensure that most of the autonomous regions were represented by at least one rheumatologist, and the panellists were selected based on sex, age, professional category and the care level of their hospital.
The entire process of constructing the CI was coordinated by a female epidemiologist with training and experience in the methodology for evaluating quality of care and CI design.
Process for Constructing the Composite IndicatorThe following stages were followed to construct the CI:
- 1.
Definition of the phenomenon to be assessed. The PI and the SC defined, through formal expert judgement, the phenomenon to be evaluated as care quality for patients with RA. Formal expert judgement is the informed opinion of people with a track record in the subject, who are recognised by others as qualified experts in the subject, and who can provide information, evidence, judgements and evaluations.
- 2.
Selection of criteria of quality, definition of their indicators and formulae. In an initial phase, the PI and the SC selected a set of 37 criteria of quality, from a review of the scientific literature, which focussed on the recommendations of the T2T strategy and on the clinical management of RA patients (preventive and health promotion measures, therapeutic management with DMDs and glucocorticoids). Table 1 shows the 37 criteria of quality that were selected; 35 of which are of process, and 2 of structure.
Table 1.Selected Quality Criteria.
1. The interval between requesting a rheumatology consultation and the first visit to the rheumatologist was less than 4 weeks 2. The interval between the first rheumatology visit and establishing a diagnosis of RA was less than 2 weeks 3. The interval between the first visit to rheumatology and starting MTX or the first conventional DMD was less than 2 weeks. 4. It is recorded in the medical history that the patient was advised to stop smoking 5. It is recorded in the medical history that the patient was advised to achieve and maintain an appropriate weight 6. It is recorded in the medical history that the patient was advised to maintain good oral health 7. It is recorded in the medical history that an explicit assessment was made of factors of poor prognosis as a determining element in the choice of treatment 8. It is recorded in the medical history that an explicit evaluation of activity was made as a determining element in the choice of treatment 9. A chest X-ray was performed before MTX 10. Folic acid was prescribed if MTX was prescribed 11. Low doses of glucocorticoids and DMDs were prescribed 12. Glucocorticoids have were not prescribed in doses above 10mg/day of prednisone or the equivalent for periods longer than one month 13. It is recorded in the medical history that injections were prescribed for inflamed joints as part of good systemic control of the disease. 14. It is recorded in the medical history that the dose of MTX was increased to 20mg/week before deciding an inadequate response to MTX, in the absence of adverse effects that made it necessary to discontinue MTX 15. The MTX administration route was recorded 16. It is recorded in the medical history that the patient was monitored every month until remission or low disease activity 17. It is recorded in the medical history that activity indices were used at visits (DAS28, SDAI, CDAI) or at least their individual components collected (VAS, joint counts, acute phase reactants) 18. It is recorded in the medical history that therapy was intensified according to the pre-established target 19. It is recorded in the medical history that the patient was informed about target-based therapy 20. The patient was prescribed double or triple therapy with conventional DMDs before prescribing the first biological agent. 21. The patient was prescribed a second conventional DMD in monotherapy or combined therapy before the first biological agent was prescribed. 22. A chest X-ray and PPD were performed before starting the first biological agent 23. HBV, HCV and HIV serologies were taken before the first biological agent 24. The patient was monitored every 6–8 months after appropriate control of the disease was achieved 25. Radiological monitoring was performed every 3–12 months 26. Other imaging techniques were performed, such as ultrasound or magnetic resonance, to monitor subclinical inflammatory activity 27. It is recorded in the medical history that the patient's comorbidities were considered when designing the treatment and setting the therapeutic target 28. High blood pressure was recorded in the medical history 29. It is recorded in the medical history that dyslipidaemia was considered 30. It is recorded in the medical history that diabetes mellitus was considered 31. It is recorded in the medical history that flu vaccination was recommended 32. It is recorded in the medical history that pneumococcal vaccination was recommended 33. It is recorded in the medical history that health staff (nurse or rheumatologist) monitored cardiovascular risk 34. The patient's weight and height were recorded 35. Telephone follow-up was made of compliance with conventional DMD treatment or MTX biological agents or biologics 36. There is a fast track pathway in the rheumatology department for suspected inflammatory disease. 37. There is an care mechanism should a stable patient suffer a flare-up RA: rheumatoid arthritis; CDAI: Clinical Disease Activity Index; DAS28: Disease Activity Score; VAS: visual analogue scale; DMD: disease-modifying drug; MTX: methotrexate; PPD: purified protein derivative; SDAI: Simple Disease Activity Index; HBV: hepatitis B virus; HCV: hepatitis C virus C; HIV: human immunodeficiency virus.
- 3.
Prioritisation of the criteria of quality. The prioritisation of the quality criteria were agreed using the Delphi methodology: a systematic, interactive and group process aimed at collecting opinions and consensus on the relevance and feasibility of the criteria of quality, based on the experience and subjective judgement of experts.7 The panel who took part in the Delphi methodology comprised the aforementioned group of experts, who agreed the grade of agreement on the clinical relevance and the feasibility of collecting data in the medical history of the evaluated criteria of quality, as well as offering suggestions or changes to the drafting of the criteria based on an open question included in the surveys. This enabled the criteria of quality to be included, removed or adjusted as proposed by the PI and the SC of the project.
The Delphi methodology was carried out in 2 rounds by prior decision. Before the first round, each of the panellists was contacted to invite them to participate and give their consent. After they had accepted, they were each sent the first round questionnaire by email, and instructions on how to complete it.
First round questionnaire. This questionnaire contained 37 criteria of quality (see supplementary material), organised in 2 blocks, according to the dimensions of quality (accessibility and scientific and technical appropriateness) and the nature of the criterion (structure and process): block A: accessibility criteria (3 criteria), block B: scientific and technical appropriateness criteria (32 criteria), and block C: structure criteria (2 criteria). Each criterion was assessed according to its relevance and feasibility, using a visual numerical scale from 0 to 10, to measure the grade of agreement with each statement. A score “0” indicted complete disagreement and a score “10” indicated complete agreement with the statement. Relevance was defined as the clinical importance of the criterion, understood as the impact on health outcomes, and feasibility as the availability of resources to evaluate the criterion of quality, i.e., if the information to assess the quality was available in the patients’ medical history, this is the source of information used to develop quality of care.
For each of the criteria of quality to be agreed the panellist was requested to justify their response from comments based on their professional experience or scientific evidence-based information.
Second round questionnaire. The second round questionnaire comprised 12 criteria and followed the same structure as the first (see supplementary material). The panellist's individual assessment was added to each criterion and the score awarded in the first round by the group of experts.
A qualitative and quantitative analysis was performed of both rounds. The quantitative analysis consisted of calculating the median, as a measure of central tendency, and the interquartile range, as a measure of dispersion. In addition, the percentage of responses was calculated with a score equal or above 7.5 for relevance or feasibility, for each criterion of quality. Agreement was defined when a median value equal or above 7 was achieved from more than 80% of the panellists. The qualitative phase of the first round consisted of analysing the comments on the criteria made by the panellists.
The analysis of the second round was performed following the same methodology and the same criteria as that used to establish agreement in the first round.
Based on the prioritised criteria of quality, the quality indicators and their formulae were defined following the methodology for the design and creation of the indicators (see Table 2).8
- 4.
Construction of the CI. The sum of the values obtained for each indicator was used as the aggregation method for constructing the CI, this is a simple method widely used in the area of health.9,10 The indicators were measured in the form of simple percentages, and the average of the medians of the relevance and feasibility assessments awarded by the panel of experts in the Delphi methodology were used as the weight factor.
Prioritised Quality Criteria, Indicators and Formulae.
| Quality criteria | Quality indicator | Formulae |
|---|---|---|
| Criterion 1. The Interval between the first rheumatology visit and establishing a diagnosis of RA was less than 2 weeks | Percentage of patients for whom the Interval between the first visit to rheumatology and diagnosis of RA was less than 2 weeks | Numerator: number of patients for whom the first consultation in the Rheumatology department and diagnosis of the disease was less than 2 weeks. Denominator: total patients * 100 |
| Criterion 2. The interval between the first rheumatology visit and starting MTX or the first conventional DMD was less than 2 weeks | Percentage of patients for whom the interval between the first rheumatology visit and starting treatment with a first DMD was less than 2 weeks | Numerator: number of patients for whom the interval between the first consultation with the rheumatology department and starting treatment with a first DMD was less than 2 weeks Denominator: total patients * 100 |
| Criterion 3. Patients with RA who are smokers should receive personalised advice on smoking cessation | Percentage of patients who are smokers whose rheumatologist offered them personalised advice on smoking cessation | Numerator: number of patients whose medical histories record that they were offered advice on smoking cessation Denominator: total patients recorded as smokers in their medical histories * 100 |
| Criterion 4. It is recorded in the medical history that the patient was advised to achieve and maintain an appropriate weight | Percentage of patients whose medical histories record that their rheumatologist offered them advice on weight control | Numerator: number of patients whose medical histories record that they were advised to achieve and maintain an appropriate weight Denominator: total patients * 100 |
| Criterion 5. It is recorded in the medical history that an explicit assessment was made of factors of poor prognosis as a determining element in the choice of treatment | Percentage of patients whose medical history records that their disease activity was explicitly evaluated by means of a joint count (number of painful joints and number of swollen joints), the patient's overall assessment using the patient's visual analogue score (VAS) and acute phase reactants (ESR and CRP) | Numerator: number of patients whose medical history records that poor prognosis factors were included Denominator: total patients * 100 |
| Criterion 6. It is recorded in the medical history that an explicit assessment of activity was made as a determining element in the choice of treatment | Percentage of patients whose medical history records that their disease activity was explicitly evaluated by means of a joint count (number of painful joints and number of swollen joints), the patient's overall assessment using the patient's visual analogue score (VAS), and acute phase reactants (ESR and CRP) | Numerator: number of patients whose medical history records that their disease activity was assessed Denominator: total patients * 100 |
| Criterion 7. A chest X-ray was performed before MTX | Percentage of patients whose medical history records that a chest X-ray was performed in the two months before starting treatment with MTX. It is considered to have been “recorded” in the medical history when their rheumatologist explicitly recorded it in writing or if there is a chest-X-ray dated before (2 months) starting treatment with MTX | Numerator: number of patients with a medical history recording that a chest X-ray was performed (within 2 months) before starting treatment with MTX Denominator: All the patients who started treatment with MTX * 100 |
| Criterion 8. Folic acid or folinic acid was prescribed if MTX has been prescribed | Percentage of patients whose medical history records that they were prescribed folic acid or folinic acid if they have been prescribed MTX | Numerator: number of patients prescribed folic acid or folinic acid Denominator: total patients starting treatment with MTX * 100 |
| Criterion 9. Glucocorticoids were not prescribed in doses above 10mg/day of prednisone or the equivalent for periods longer than one month. Methylprednisolone at a dose of 8mg/day is considered a glucocorticoid equivalent to prednisone | Percentage of patients who were not prescribed glucocorticoids in doses above 10mg/day of prednisone or methylprednisolone at doses above 8mg/day for a period exceeding one month | Numerator: number of patients who were not prescribed glucocorticoids at doses above 10mg/day of prednisone or methylprednisolone at 8mg/day during a period exceeding one month Denominator: total patients prescribed glucocorticoids * 100 |
| Criterion 10. It is recorded in the medical history that the dose of MTX was increased to 20mg/week before deciding an inadequate response to MTX, in the absence of adverse effects that made it necessary to discontinue MTX | Percentage of patients whose dose of MTX was increased to 20mg/week before an inadequate response to MTX was decided, in the absence of adverse effects making it necessary to discontinue the drug. An inadequate response is determined by clinical judgement. The adverse effects associated with MTX are: alopecia, nausea, vomiting, diarrhoea, ulcerative stomatitis, oral aphtha, gingivitis, glossitis, abdominal discomfort, sexual impotence in males, reduced libido, headache and dizziness | Numerator: number of patients whose medical history records that their dose of MTX was increased to 20mg/week Denominator: total patients with an inadequate response to MTX, in the absence of adverse effects that made it necessary to discontinue treatment with MTX * 100 |
| Criterion 11. The MTX administration route was recorded | Percentage of patients whose medical history records the administration route of MTX | Numerator: number of patients whose medical history records the administration route of MTX Denominator: total pacientes prescribed MTX * 100. In order to assess this indicator all the times that MTX was administered over the study period will be considered |
| Criterion 12. It is recorded in the medical history that the patient was monitored every month until remission or low disease activity according to DAS28, CDAI, SDAI or EULAR remission criteria | Percentage of patients whose medical history records that they were monitored using one of the following indices (DAS28, CDAI, SDAI) or EULAR criteria every 4 weeks, until remission or low disease activity. Clinical remission is considered with DAS28<2.4, CDAI<2.8 or SDAI<3.3, and low disease activity DAS28<3.2, CDAI=2.8–10 or SDAI<11. The definition of remission according to EULAR criteria can be categorical, when all of the following must be met: painful joint≤1, swollen joints≤1, CRP≤1mg/dl, overall patient's assessment≤1, on a scale of 0–10, or can be based on an index (SDAI≤3.3) | Numerator: number of patients whose disease activity was monitored using DAS28, CDAI, SDAI, every 4 weeks, from the start of treatment Denominator: total patients whose disease was not monitored (using DAS28>3.2 or SDAI>11) before starting disease-modifying treatment and achieving remission or low disease activity * 100 The disease-modifying treatment included conventional DMDs (MTX, LFN, SFZ and HCQ) and biological therapy (INF, ETN, ADA, CERTO, GOL, ABA, TCZ, RTX) |
| Criterion 13. It is recorded in the medical history that injections were prescribed for inflamed joints as part of good systemic control of the disease | Percentage of patients with good systemic control of their disease prescribed injections for inflamed joints | Numerator: number of patients with good systemic control Denominator: total patients prescribed injection of painful joints * 100 |
| Criterion 14. It is recorded in the medical history that activity indices were used in visits or at least their individual components collected | Percentage of patients whose medical history records the indices of disease activity (DAS28, SDAI, CDAI) or at least all the individual components (VAS, joint counts, acute phase reactants. | Numerator: number of patients whose medical history records their disease activity or individual components Denominator: total patients * 100 |
| Criterion 15. It is recorded in the medical history that therapy was intensified according to the pre-established target | Percentage of patients whose medical history records that their therapy was modified when low activity or remission of their disease was not achieved. Clinical remission is considered DAS28<2.4, CDAI<2.8 or SDAI<3.3, and low disease activity DAS28<3.2, CDAI=2.8–10 or SDAI<11. The definition of remission according to EULAR criteria can be categorical, requiring all of the following to be met: painful joint≤1, inflamed joints≤1, CRP ≤ 1mg/dl or the patient's assessment of the activity≤1, on a scale of 0–10 | Numerator: number of patients whose medical history records that their therapy was modified because low activity or remission of their disease was not achieved. Denominator: total patients with RA * 100 |
| Criterion 16. The patient was prescribed a second conventional DMD in monotherapy or combined therapy before prescribing the first biological agent | Percentage of patients prescribed a second conventional DMD in monotherapy or combined therapy before prescribing the first biological agent. The conventional DMDs are: MTX, LFN, SFZ, HCQ | Numerator: number of patients prescribed a second conventional DMD in monotherapy or combined therapy prior to treatment with biologicals Denominator: total patients prescribed treatment with a biological agent for the first time * 100 |
| Criterion 17. A chest X-ray and PPD were performed before starting the first biological agent | Percentage of patients who underwent a chest X-ray and PPD (PPD skin test to diagnose tuberculosis infection) before starting the first biological agent. | Numerator: number of patients who underwent HCV, HBV and HIV serologies before starting the first biological Denominator: total patients prescribed biological therapy for the first time * 100 |
| Criterion 18. HBV, HCV and HIV serologies were taken before the first biological agent | Percentage of patients whose medical history records that serologies were taken (HCV, HBV and HIV) before starting biological therapy | Numerator: number of patients who underwent HCV, HBV and HIV serologies dated prior to starting the first biological Denominator: total patients prescribed biological therapy for the first time * 100 |
| Criterion 19. The patient was monitored every 6–8 months after appropriate control of the disease was achieved | Percentage of patients whose medical history records that they attended a check-up at 6–8 months or earlier after appropriate control of the disease. Appropriate control of the disease is defined as DAS28<3.2 or SDAI<11 | Numerator: number of patients who attended check-up at 6–8 months or after appropriate control of the disease Denominator: total number of patients with appropriate control of their disease * 100 |
| Criterion 20. Radiological monitoring was performed at least once a year | Percentage of patients whose medical history records that a hand X-ray was taken at least once in 12 months | Numerator: number of patients who undergo a hand X-ray, at least once a year Denominator: total patients with RA * 100 |
| Criterion 21. The medical history records that the patient's comorbidities were considered when designing treatment and setting the therapeutic target | Percentage of patients whose medical history records that a comorbidity affected the choice of a specific RA treatment | Numerator: numbers of patients whose medical history records comorbidities (high blood pressure, diabetes, etc.) Denominator: total patients RA * 100 |
| Criterion 22. High blood pressure was recorded in the medical history | Percentage of patients whose medical history records blood pressure | Numerator: number of patients whose medical histories record high blood pressure Denominator: total patient with RA * 100 |
| Criterion 23. It is recorded in the medical history that dyslipidaemia was considered | Percentage of patients whose medical history records their cholesterol level | Numerator: number of patients whose medical histories record their cholesterol level Denominator: total patients with RA * 100 |
| Criterion 24. It is recorded in the medical history that diabetes mellitus was considered | Percentage of patients whose medical history records their blood sugar level | Numerator: number of patients whose medical histories record their blood sugar level Denominator: total patients with RA * 100 |
| Criterion 25. It is recorded in the medical history that annual flu vaccination was recommended | Percentage of patients whose medical history records that annual flu vaccination was recommended | Numerator: number of patients recommended to have an annual flu vaccine Denominator: total patients with RA * 100 |
| Criterion 26. It is recorded in the medical history that pneumococcal vaccination was recommended | Percentage of patients whose medical history records that pneumococcal vaccination was recommended | Numerator: number of patients recommended pneumococcal vaccination Denominator: total patients with RA * 100. |
| Criterion 27. It is recorded in the medical history that health staff (nurse or rheumatologist) monitored cardiovascular risk | Percentage of patients whose medical history records that health staff (nurse or rheumatologist) monitored their cardiovascular risk using a cardiovascular risk scale or subclinical atherosclerosis testing | Numerator: number of patients whose cardiovascular risk was assessed using a cardio vascular risk scale or subclinical atherosclerosis testing Denominator: total patients with RA * 100 |
| Criterion 28. The patient's weight was recorded in their medical history | Percentage of patients whose medical history records an annual weight measurement | Numerator: number of patients whose medical history records an annual measurement of their weight Denominator: total patients * 100 |
| Criterion 29. The patient's height was recorded in their medical history | Percentage of patients whose medical history records their height measurement | Numerator: number of patients whose medical history records their height Denominator: total patients * 100 |
| Criterion 30. There is a fast- track pathway in the rheumatology department for suspected inflammatory disease | Presence of a fast- track pathway in the rheumatology department for suspected inflammatory disease | |
| Criterion 31. There is a care mechanism should a stable patient suffer a flare-up | Presence of a care mechanism in the event of a flare-up of stable RA |
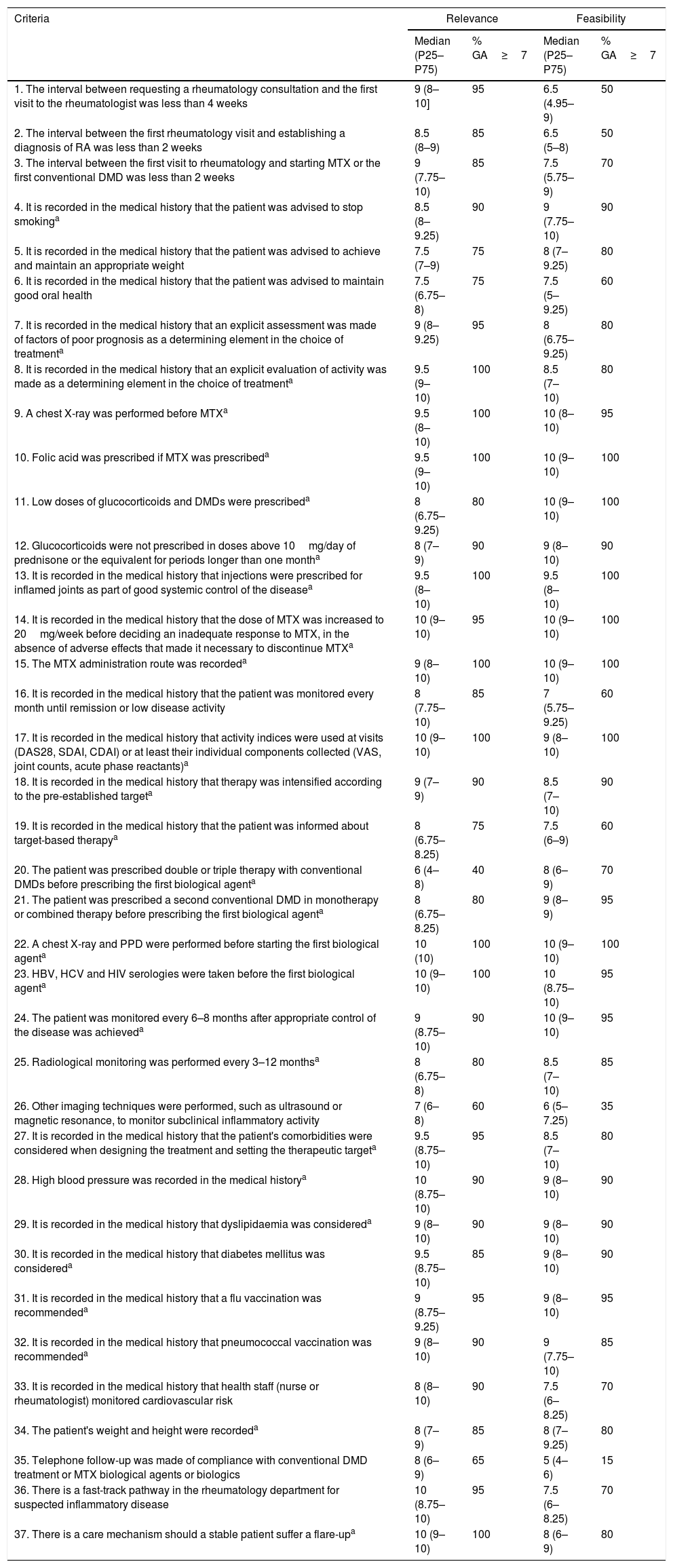
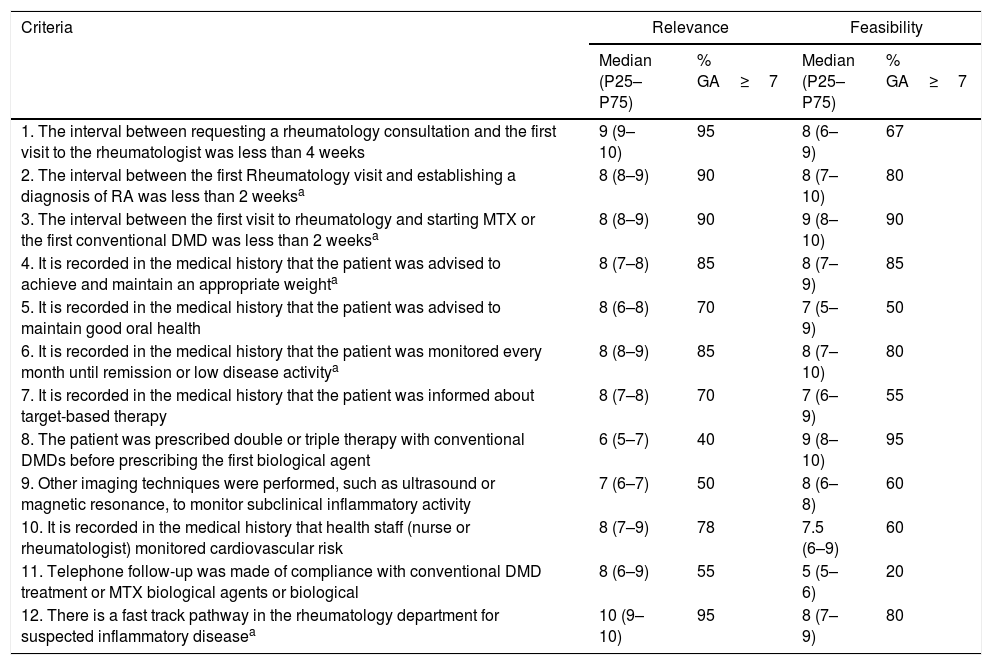
Of the 37 criteria selected, 12 passed through to the second Delphi round. After the analysis of this round, the prioritised quality indicators and, therefore, forming part of the CI, totalled 31. The results of the first and second Delphi rounds are shown in Tables 3 and 4.
Priorisation of Quality Criteria. First Delphi Round.
| Criteria | Relevance | Feasibility | ||
|---|---|---|---|---|
| Median (P25–P75) | % GA≥7 | Median (P25–P75) | % GA≥7 | |
| 1. The interval between requesting a rheumatology consultation and the first visit to the rheumatologist was less than 4 weeks | 9 (8–10] | 95 | 6.5 (4.95–9) | 50 |
| 2. The interval between the first rheumatology visit and establishing a diagnosis of RA was less than 2 weeks | 8.5 (8–9) | 85 | 6.5 (5–8) | 50 |
| 3. The interval between the first visit to rheumatology and starting MTX or the first conventional DMD was less than 2 weeks | 9 (7.75–10) | 85 | 7.5 (5.75–9) | 70 |
| 4. It is recorded in the medical history that the patient was advised to stop smokinga | 8.5 (8–9.25) | 90 | 9 (7.75–10) | 90 |
| 5. It is recorded in the medical history that the patient was advised to achieve and maintain an appropriate weight | 7.5 (7–9) | 75 | 8 (7–9.25) | 80 |
| 6. It is recorded in the medical history that the patient was advised to maintain good oral health | 7.5 (6.75–8) | 75 | 7.5 (5–9.25) | 60 |
| 7. It is recorded in the medical history that an explicit assessment was made of factors of poor prognosis as a determining element in the choice of treatmenta | 9 (8–9.25) | 95 | 8 (6.75–9.25) | 80 |
| 8. It is recorded in the medical history that an explicit evaluation of activity was made as a determining element in the choice of treatmenta | 9.5 (9–10) | 100 | 8.5 (7–10) | 80 |
| 9. A chest X-ray was performed before MTXa | 9.5 (8–10) | 100 | 10 (8–10) | 95 |
| 10. Folic acid was prescribed if MTX was prescribeda | 9.5 (9–10) | 100 | 10 (9–10) | 100 |
| 11. Low doses of glucocorticoids and DMDs were prescribeda | 8 (6.75–9.25) | 80 | 10 (9–10) | 100 |
| 12. Glucocorticoids were not prescribed in doses above 10mg/day of prednisone or the equivalent for periods longer than one montha | 8 (7–9) | 90 | 9 (8–10) | 90 |
| 13. It is recorded in the medical history that injections were prescribed for inflamed joints as part of good systemic control of the diseasea | 9.5 (8–10) | 100 | 9.5 (8–10) | 100 |
| 14. It is recorded in the medical history that the dose of MTX was increased to 20mg/week before deciding an inadequate response to MTX, in the absence of adverse effects that made it necessary to discontinue MTXa | 10 (9–10) | 95 | 10 (9–10) | 100 |
| 15. The MTX administration route was recordeda | 9 (8–10) | 100 | 10 (9–10) | 100 |
| 16. It is recorded in the medical history that the patient was monitored every month until remission or low disease activity | 8 (7.75–10) | 85 | 7 (5.75–9.25) | 60 |
| 17. It is recorded in the medical history that activity indices were used at visits (DAS28, SDAI, CDAI) or at least their individual components collected (VAS, joint counts, acute phase reactants)a | 10 (9–10) | 100 | 9 (8–10) | 100 |
| 18. It is recorded in the medical history that therapy was intensified according to the pre-established targeta | 9 (7–9) | 90 | 8.5 (7–10) | 90 |
| 19. It is recorded in the medical history that the patient was informed about target-based therapya | 8 (6.75–8.25) | 75 | 7.5 (6–9) | 60 |
| 20. The patient was prescribed double or triple therapy with conventional DMDs before prescribing the first biological agenta | 6 (4–8) | 40 | 8 (6–9) | 70 |
| 21. The patient was prescribed a second conventional DMD in monotherapy or combined therapy before prescribing the first biological agenta | 8 (6.75–8.25) | 80 | 9 (8–9) | 95 |
| 22. A chest X-ray and PPD were performed before starting the first biological agenta | 10 (10) | 100 | 10 (9–10) | 100 |
| 23. HBV, HCV and HIV serologies were taken before the first biological agenta | 10 (9–10) | 100 | 10 (8.75–10) | 95 |
| 24. The patient was monitored every 6–8 months after appropriate control of the disease was achieveda | 9 (8.75–10) | 90 | 10 (9–10) | 95 |
| 25. Radiological monitoring was performed every 3–12 monthsa | 8 (6.75–8) | 80 | 8.5 (7–10) | 85 |
| 26. Other imaging techniques were performed, such as ultrasound or magnetic resonance, to monitor subclinical inflammatory activity | 7 (6–8) | 60 | 6 (5–7.25) | 35 |
| 27. It is recorded in the medical history that the patient's comorbidities were considered when designing the treatment and setting the therapeutic targeta | 9.5 (8.75–10) | 95 | 8.5 (7–10) | 80 |
| 28. High blood pressure was recorded in the medical historya | 10 (8.75–10) | 90 | 9 (8–10) | 90 |
| 29. It is recorded in the medical history that dyslipidaemia was considereda | 9 (8–10) | 90 | 9 (8–10) | 90 |
| 30. It is recorded in the medical history that diabetes mellitus was considereda | 9.5 (8.75–10) | 85 | 9 (8–10) | 90 |
| 31. It is recorded in the medical history that a flu vaccination was recommendeda | 9 (8.75–9.25) | 95 | 9 (8–10) | 95 |
| 32. It is recorded in the medical history that pneumococcal vaccination was recommendeda | 9 (8–10) | 90 | 9 (7.75–10) | 85 |
| 33. It is recorded in the medical history that health staff (nurse or rheumatologist) monitored cardiovascular risk | 8 (8–10) | 90 | 7.5 (6–8.25) | 70 |
| 34. The patient's weight and height were recordeda | 8 (7–9) | 85 | 8 (7–9.25) | 80 |
| 35. Telephone follow-up was made of compliance with conventional DMD treatment or MTX biological agents or biologics | 8 (6–9) | 65 | 5 (4–6) | 15 |
| 36. There is a fast-track pathway in the rheumatology department for suspected inflammatory disease | 10 (8.75–10) | 95 | 7.5 (6–8.25) | 70 |
| 37. There is a care mechanism should a stable patient suffer a flare-upa | 10 (9–10) | 100 | 8 (6–9) | 80 |
RA: rheumatoid arthritis; CDAI: Clinical Disease Activity Index; DAS: Disease Activity Score; VAS: visual analogue scale; DMD: disease modifying drugs; MTX: methotrexate; PPD: purified protein derivative; SDAI: Simple Disease Activity Index; HBV: hepatitis B virus; HCV: hepatitis C virus C; HIV: human immunodeficiency virus.
Priorisation of Quality Criteria. Second Delphi Round.
| Criteria | Relevance | Feasibility | ||
|---|---|---|---|---|
| Median (P25–P75) | % GA≥7 | Median (P25–P75) | % GA≥7 | |
| 1. The interval between requesting a rheumatology consultation and the first visit to the rheumatologist was less than 4 weeks | 9 (9–10) | 95 | 8 (6–9) | 67 |
| 2. The interval between the first Rheumatology visit and establishing a diagnosis of RA was less than 2 weeksa | 8 (8–9) | 90 | 8 (7–10) | 80 |
| 3. The interval between the first visit to rheumatology and starting MTX or the first conventional DMD was less than 2 weeksa | 8 (8–9) | 90 | 9 (8–10) | 90 |
| 4. It is recorded in the medical history that the patient was advised to achieve and maintain an appropriate weighta | 8 (7–8) | 85 | 8 (7–9) | 85 |
| 5. It is recorded in the medical history that the patient was advised to maintain good oral health | 8 (6–8) | 70 | 7 (5–9) | 50 |
| 6. It is recorded in the medical history that the patient was monitored every month until remission or low disease activitya | 8 (8–9) | 85 | 8 (7–10) | 80 |
| 7. It is recorded in the medical history that the patient was informed about target-based therapy | 8 (7–8) | 70 | 7 (6–9) | 55 |
| 8. The patient was prescribed double or triple therapy with conventional DMDs before prescribing the first biological agent | 6 (5–7) | 40 | 9 (8–10) | 95 |
| 9. Other imaging techniques were performed, such as ultrasound or magnetic resonance, to monitor subclinical inflammatory activity | 7 (6–7) | 50 | 8 (6–8) | 60 |
| 10. It is recorded in the medical history that health staff (nurse or rheumatologist) monitored cardiovascular risk | 8 (7–9) | 78 | 7.5 (6–9) | 60 |
| 11. Telephone follow-up was made of compliance with conventional DMD treatment or MTX biological agents or biological | 8 (6–9) | 55 | 5 (5–6) | 20 |
| 12. There is a fast track pathway in the rheumatology department for suspected inflammatory diseasea | 10 (9–10) | 95 | 8 (7–9) | 80 |
RA: rheumatoid arthritis; DMD: disease-modifying drugs; MTX: methotrexate
The formula for calculating the weighted CI (WCI) was:
(Ind=indicator)
DiscussionThis study enabled the construction of a CI that offers a synthetic measure of the quality of care for patients with RA in the RU of Spanish hospitals, and a way of monitoring so that a check can be made that patients are being attended within the pre-established ranges defining quality of care, and that any problems that require action can be identified.11
The use of this type of indicator that summarises the measurement of various aspects of the same problem in a single number,12 is widely known in socioeconomic13,14 and health15,16 environments. In the field of rheumatology, these indicators are also used a great deal as synthetic measures, essentially to evaluate clinical factors, such as the inflammatory activity of many diseases (DAS28,17 BASDAI18 and CDAI19), and are an essential pillar in their management and control. In contrast, there is far less experience in the use of these CI to evaluate more general concepts, such as quality of care in rheumatology. A strength of this paper is that it provides the construction of an indicator that enables an assessment of the scientific and technological appropriateness deriving from the T2T strategy (evaluation of inflammatory activity and pharmacological guidance to achieve the therapeutic target), general recommendations on healthy life habits, preventative measures, pharmacological management with traditional DMDs and glucocorticoids for the control of RA, and organisational factors of the units.
Some of the advantages of using these indicators is that, as they provide an overall view of the quality of care, which makes them more interpretable than the individual assessment of a set of indictors, they are of great interest for the public in general, and provide a measure that enable comparisons with other units, such as for example other healthcare systems, clinical services or countries (European Union Consultative meeting held in Brussels in 200214). Another aspect in favour of the constructed CI is not reconverting the indicator scales (29 of the 31 indicators that comprise the CI use the same measurement system), which also makes this an easily interpretable CI.
Although they have major strengths, it is worth mentioning that these indicators also have some weaknesses, essentially arising from the methodology used for their construction: the selection of the indicators, the grouping methodology or the need for a great amount of data for their construction, since all the component indicators have to be measured.12 This, although still relevant since it would affect the validity of the indicator, is considered a methodological challenge to resolve more than a real disadvantage that would limit their use. In this regard, in this paper the construction of the CI followed a rigorous methodology so as not to affect its internal validity, and for it to be easily interpretable. The selections of quality criteria were based on the scientific literature and on the clinical experience of rheumatologists with a wide knowledge of the management of patients with RA. Just as efforts were made to achieve the representativeness of the panellists who took part in the selection and the prioritisation of the criteria in terms of sociodemographic features, years of care activity, professional category and the care level of their centre.
A limit that is worthy of mention is the aggregation method used to construct the CI. Using the sum made it simple to apply and interpret, although as occurs with other CI that use this method (Top 20 Classification of Spain's hospitals),9 the mean values can mask situations of very low indicators which are compensated by others that are higher. Another limitation to flag up with these CI is that, although all the indicators that construct them are measurable, some of them could be over-represented, because other indicators have been under-recorded due to a lack of information in the medical history. Nevertheless, this is not expected to be particularly relevant in the CI of this study, since, in the criteria prioritisation phase, the group of experts explored the feasibility of evaluating the indicators using a two-round Delphi, taking into account that there was enough information in the medical histories. Those which the experts considered, by consensus, were not feasible to evaluate because there was a high probability of a lack of information in the medical histories did not go on to form part of the final list of criteria to be assessed.
Regarding the weighting, we should mention that despite having constructed the CI as a weighted index, in practice it behaved like an unweighted index, since there was hardly any variability in the magnitude of the weighting factor of the indicators, and they were considered not to be discriminating in relevance and feasibility. The weighting method used, the agreement in expert judgement, although accepted for its validity and widely used to weight indicators by other international organisations,16,20 requires further studies that use different weighting methods with a view to confirming these findings.
From a clinical and care perspective, the availability of this indicator and its individual components will enable areas for improvement to be identified in the care process of a disease such as RA, where it is widely accepted that speedy diagnosis and treatment with disease-modifying drugs, dynamic treatment adjustment and the goal of remission or, at least low disease activity, result in reduced structural damage and functional limitation for patients.
In conclusion, this study presents the development of a CI of care quality for RA patients, providing an objective and agreed tool for overall assessment of the care process.
Ethical DiclosuresProtection of people and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FinancingThe project promoter is the Spanish Rheumatology Foundation with funds awarded by Abbvie to finance the ARExcellence Project. The company did not participate in the design of the Project or intervene in the conclusions or the drawing up of the final report or this manuscript.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Martín-Martínez MA, Andreu-Sanchez JL, Sanchez-Alonso F, Corominas H, Perez-Venegas JJ, Roman-Ivorra JA, et al. Indicador compuesto para evaluar la calidad asistencial en el manejo de los pacientes con artritis reumatoide en las consultas externas de Reumatología. Reumatol Clin. 2019;15:156–164.