Cryoglobulins are immunoglobulins that precipitate at cold temperatures. Their presence can be related to a type of vasculitis referred to as cryoglobulinemia. This condition, especially mixed cryoglobulinemia, has been associated with viral infections like hepatitis C virus in 60%–90% of cases, but it has also been reported in relation to connective tissue diseases, usually resulting in a more severe course. We describe the case of a patient with seronegative polyarthritis who developed acute arterial ischemia in association with cryoglobulinemia, with a good response to rituximab therapy.
Las crioglobulinas son inmunoglobulinas que precipitan con el frío. Su presencia puede asociarse a un tipo de vasculitis denominada crioglobulinemia. Estas, especialmente las mixtas, se asocian a infecciones como el virus de la hepatitis C hasta en el 60–90% de los casos, aunque también se ha descrito su asociación a enfermedades del tejido conectivo, en las que suele tener un curso más agresivo. Se presenta el caso de una paciente con poliartritis seronegativa que desarrolló isquemia arterial aguda en el contexto de una crioglobulinemia y que ha respondido al tratamiento con rituximab.
Polyarthritis is a common reason for seeing the doctor and is a challenge for rheumatologists because of its extensive differential diagnosis. In clinical practice, the development of new symptoms and analytical findings during patient follow-up can lead to a change in the diagnosis and, thus, of the treatment begun.
Cryoglobulinemia is a vasculitis characterized by the involvement of small and medium-sized vessels. Three types of cryoglobulinemia have been described, types II and III being those that are associated with connective tissue diseases (CTD). The clinical manifestations of cryoglobulinemia can range from nonspecific symptoms to renal, neuropathic, joint and skin involvement. This means that, on occasion, these symptoms can be confounded with or overlap those linked to the CTD with which they are associated.1
We report the case of a patient whose presenting symptom was polyarthritis and who, during follow-up, was diagnosed with cryoglobulinemia associated with systemic lupus erythematosus (SLE), with a good response to immunosuppressive therapy with rituximab.
Clinical ObservationThe patient was a 41-year-old Paraguayan woman who was noteworthy only in that she smoked 40 cigarettes/day. She presented with a 2-month history of polyarthritis in hands, feet and ankles. Treatment was initiated with nonsteroidal anti-inflammatory drugs and prednisone, and she was scheduled to undergo a complete study of polyarthritis. Laboratory tests showed a C-reactive protein (CRP) of 24.5mg/dL and an erythrocyte sedimentation rate (ESR) of 34mm/h; tests for rheumatoid factor and anti-cyclic citrullinated peptide were negative; she had an antinuclear antibody (ANA) titer of 1:80 with a speckled pattern and was negative for anti-extractable nuclear antigen (ENA) antibodies. Thyroid hormones, liver function and renal function were normal. Mantoux and booster, and serological testing for hepatitis B and C and human immunodeficiency virus were negative. Radiographs of hands, feet and thorax were normal. Despite the treatment, arthritis persisted in hands, carpal bones, feet and ankles. With the diagnosis of seronegative polyarthritis, treatment was begun with methotrexate (MTX) at 10mg/week and hydroxychloroquine (HCQ), with a partial response and, thus, the latter was interrupted and leflunomide was added, with good control of the disease for more than a year.
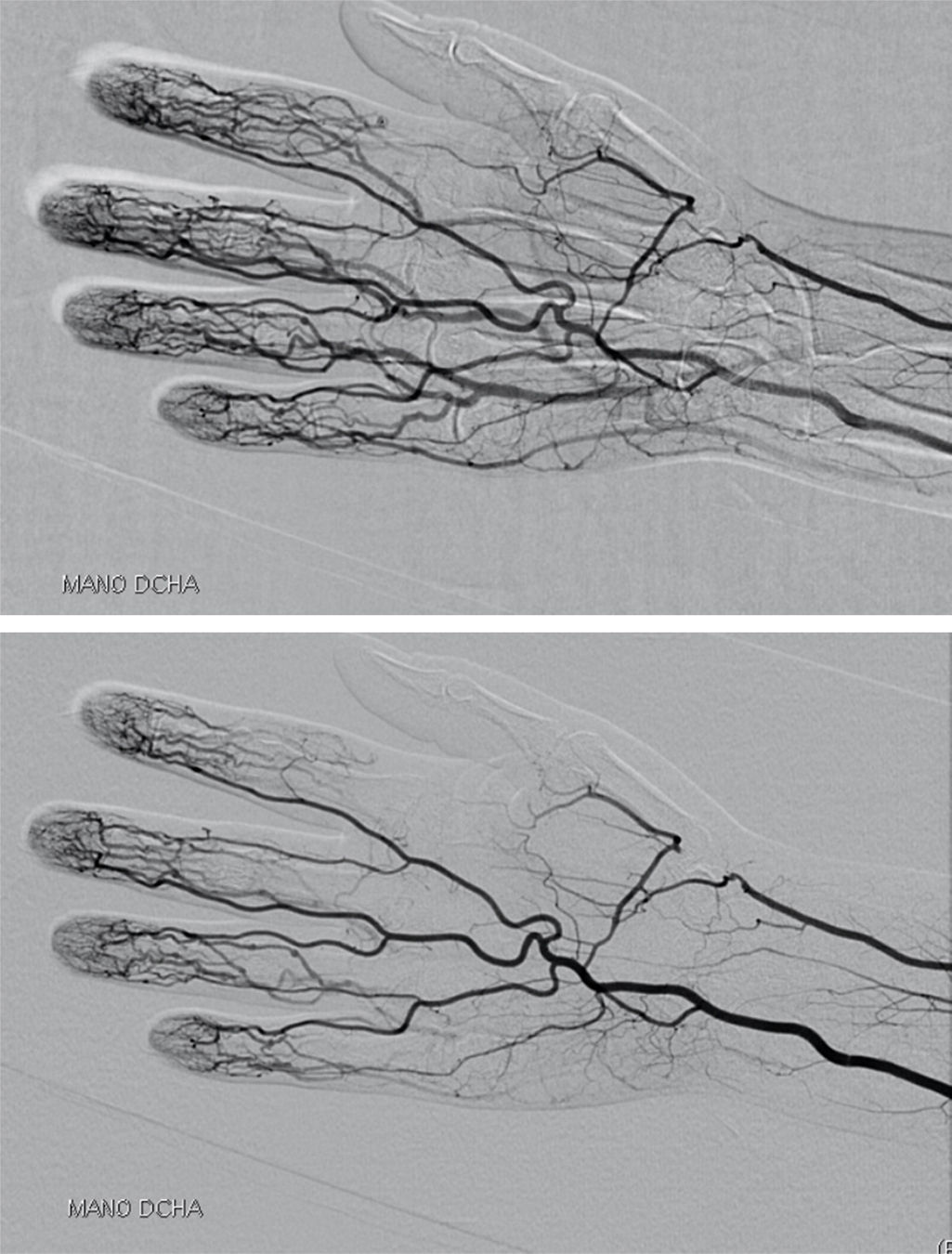
The patient stopped coming to our office. In October of 2012, she suddenly began to detect cyanosis and cold in the distal phalanx of the index finger of her right hand, and she was admitted to the hospital. She underwent computed tomography angiography, which showed an occlusion of the right radial artery at the level of the anatomical snuffbox; the rest of the study (including supra-aortic trunks, study of upper limb arteries, lower limb arteries and abdominal aortography) was normal, and there were no signs of vasculitis (Fig. 1). Electrocardiogram and echocardiogram were normal. Laboratory tests revealed iron-deficiency anemia (hemoglobin 8.3mg/dL, hematocrit 27%, iron 12μg/dL, transferrin 339mg/dL, ferritin 4ng/mL), leukocytes 4250/μL, lymphocytes 510/μL (12%), neutrophils 3620/μL (85%) and platelets 359,000mm−1.3 The coagulation study was normal (prothrombin activity 118% [normal 70–120] and activated partial thromboplastin time 29.7s [23–36]). Immunoglobulin (Ig) G and IgM anticardiolipin antibodies and lupus anticoagulant were negative, and CRP and ESR were normal, as were thyroid function tests. Direct Coombs was negative and haptoglobin normal at 159mg/dL (30–200). She was positive for ANA and anti-double stranded DNA (dsDNA) and negative for ENA and antineutrophil cytoplasmic antibodies; complement C3 and C4 were normal, and proteins normal. During her hospital stay, treatment was begun with low-molecular weight heparin with acetyl salicylic acid (ASA) and intravenous alprostadil. The corticosteroid dose was increased and she continued with MTX and leflunomide (which she had continued to take although she did not come for checkups). She was discharged from the hospital, while awaiting the results of an analysis for cryoglobulins.
In successive checkups in our offices, the patient showed improvement in terms of ischemia but polyarthritis persisted. The result for cryoglobulins was positive. Given the persistence of polyarthritis (although she continued to take MTX and leflunomide) and cryoglobulinemia, it was decided that she begin rituximab. Anticoagulation was maintained with acenocoumarol for a year, until the cryoglobulins were negative, when antiplatelet therapy was begun with ASA. During follow-up, the patient was repeatedly positive for ANA at low titers, with a homogeneous pattern, and was negative for anti-dsDNA. The immunological study was extended and we found antinucleosome antibodies and antibodies against the collagen-like region of C1q. The patient has continued to take rituximab, MTX, HCQ and ASA. She has not had any further ischemic episodes and there is no evidence of involvement of kidneys or of any other organ.
DiscussionWe report the case of a patient with polyarthritis, a frequent cause for seeking advice from a rheumatologist, and a challenge for the latter because of its extensive differential diagnosis. Initially, given the clinical features and the findings revealed by the ancillary tests performed, the patient was diagnosed with rheumatoid arthritis, according to the 2010 classification criteria of the American College of Rheumatology and European League Against Rheumatism (ACR/EULAR).2 Thus, treatment with disease-modifying antirheumatic drugs (DMARD) and corticosteroids was begun, and her clinical course was favorable for more than a year. However, the development of new symptoms and analytical data recorded during follow-up, can make you reconsider the initial diagnosis or even change it, as occurred in the case we present. Ultimately, the patient met the criteria for a diagnosis of SLE, as she was ANA-positive and anti-dsDNA-positive and had arthritis, lymphopenia (according to the 2012 criteria of the Systemic Lupus International Collaborating Clinics)3 and mixed cryoglobulinemia.
We found antinucleosome and anti-C1q antibodies in this patient. The antinucleosome antibodies could be a more accurate marker for the diagnosis of SLE,4,5 with a similar specificity and a slightly higher sensitivity than anti-dsDNA, but with a highly superior diagnostic odds ratio (41 vs 27.8).5 Nucleosomes mediate the binding of autoantibodies to the basement membranes. These antibodies are capable of binding to different components of the glomerular basement membrane like collagen, membrane surface proteins, DNA-histone complexes and chromatin,6 producing renal damage. For this, they are considered nephritogenic, and have also been associated with disease activity measured using different questionnaires (Systemic Lupus Erythematosus Disease Activity Index, European Consensus Lupus Activity Measurement, etc.). The activation of the classical complement pathway begins with the molecule C1q, which is essential in the clearance of immune complexes and apoptotic cell debris. Hereditary C1q deficiency is associated with high susceptibility for the development of SLE, especially the mucocutaneous manifestations.7 Its level usually decreases during flares of the disease and is associated with anti-C1q antibodies (antibodies against the collagen-like region), especially in patients with active lupus nephritis.8 The presence of anti-DNA and anti-C1q antibodies leads to the formation of immune complexes, with the subsequent triggering of the immune response, which is usually associated with a higher risk of renal involvement.9,10 However, the expression of these antibodies and their levels do not always correlate with disease activity and, thus, their pathogenic role is not yet completely understood.11 Nevertheless, the patient has not had renal involvement over the nearly 5 years of follow-up, possibly because of the treatment with DMARD and, ultimately, with anti-CD20 monoclonal antibodies. In any case, the use of corticosteroids and immunosuppressive therapy may have contributed to the maintenance of negative anti-dsDNA antibodies throughout her course.
Moreover, the presence of cryoglobulins coincided in time with the autoimmune conditions described above. Cryoglobulins are monoclonal and/or polyclonal Ig that reversibly precipitate when serum is incubated at temperatures below 37°C.12 Their presence in blood can produce a systemic vasculitic process affecting small and medium-sized vessels. They are classified according to 3 forms depending on the composition of the precipitate:
- -
Type I cryoglobulinemia involves a single type of monoclonal immunoglobulin. It is usually associated with lymphoproliferative diseases and multiple myeloma.
- -
Type II cryoglobulinemia involves a polyclonal IgG and a monoclonal IgM with rheumatoid factor activity.
- -
Type III cryoglobulinemia involves a polyclonal IgG and a polyclonal IgM with rheumatoid factor activity.
Types II and III are referred to as mixed cryoglobulinemia (MC) and are usually associated with CTD and lymphoproliferative processes and infections, but hepatitis C virus infection is the disease with which they are most frequently linked (60%–90% of cases).13
Cryoglobulinemia is usually manifested with fever, myalgia, arthralgia (arthritis alone has only been reported in less than 10% of the cases), palpebral purpura in lower limbs (the most characteristic manifestation) and neurological and renal involvement. Regarding the last two, 17%–60% of the patients will have peripheral neuropathy, which can be the first sign of a MC, and up to 30% will develop microhematuria and proteinuria.14 Its presentation as a peripheral acute arterial ischemia, with no other associated signs of vasculitis, as occurred in our patient,12 is exceptional. The digital necrosis is adverse in the prognosis of MC, as it has been associated with a high risk of infections, sepsis and death.15 Computed tomography angiography did not identify lesions compatible with vasculitic or atherosclerotic involvement, and the patient did not have any of the abovementioned symptoms.
The pathogenic role of antinucleosome and anti-C1q antibodies in the case reported is unknown to us. We consider that they are serological markers of SLE.
The final diagnosis was MC secondary to a CTD (SLE). The usual clinical signs are the consequence of the deposition of circulating immune complexes in the wall of small and medium-sized vessels, which triggers an inflammatory response that leads to systemic vasculitis.16 Treatment consists of glucocorticoids and immunosuppressive agents. The use of plasmapheresis has been reported when the remaining approaches have been ineffective.1 In this case, given the clinical manifestations of the patient and the association of MC with SLE, we opted for rituximab. The utilization of anti-CD20 monoclonal antibodies has been found to be beneficial in the treatment of systemic vasculitis and, although the data we have in cryoglobulinemic vasculitis not associated with hepatitis C virus is limited, certain case series support the use of rituximab in these patients.17
In conclusion, the particular feature of this case lies in the presentation of the MC in the setting of SLE, as an acute arterial ischemia (right radial artery occlusion) in a patient who had initially been diagnosed as having a seronegative polyarthritis. Despite the presence of nephritogenic antibodies, the patient has not developed renal involvement, remains in remission and continues to take rituximab.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Soro Marín S, Júdez Navarro E, Alamillo Sanz AS, Sánchez Nievas G. Isquemia arterial aguda en paciente con poliartritis. Reumatol Clin. 2017;13:110–112.