SARS-CoV-2 is a new RNA virus which causes coronavirus disease 2019 (COVID-19), declared a pandemic by the World Health Organization (WHO). It triggers an atypical pneumonia that can progress to multiorgan failure. COVID-19 can cause dysregulation of the immune system, triggering an inflammatory response, and simulate haemophagocytic lymphohistiocytosis. Several studies have proposed that anti-IL-6 receptor antibodies, such as tocilizumab, play an important role in the treatment of severe acute respiratory infection associated with SARS-CoV-2. However, the role of anti-IL-1 receptor antibodies, such as anakinra, in the treatment of COVID-19 has not been established.
We present a case report of a 51-year-old man diagnosed with severe respiratory infection associated with SARS-CoV-2 that was refractory to antiviral and anti-IL-6 treatment, with a favourable clinical outcome and analytical improvement after treatment with anti-IL-1 (anakinra).
El virus SARS-CoV-2 es un nuevo virus RNA causante de la enfermedad COVID-19, declarada como pandemia por la Organización Mundial de la Salud (OMS). Produce un cuadro de neumonía atípica que puede desembocar en un fallo multiorgánico. La desregulación del sistema inmune secundaria a la infección produce un cuadro similar al síndrome de linfohistiocitosis hemofagocítica (SLHH). Varios estudios han definido la importancia que los inhibidores de la IL-6 (Tocilizumab) tienen en el tratamiento de la infección por SARS-CoV-2, sin embargo, la indicación de tratamiento con inhibidores de IL-1 (anakinra) no se encuentra establecida de forma clara.
Presentamos el caso de un paciente de 51 años con neumonía bilateral secundaria a infección por SARS-CoV-2 refractaria al tratamiento antiviral y anti-IL-6 que presentó mejoría clínica y analítica tras el tratamiento con anti-IL-1 (anakinra).
The SARS-CoV-2 virus is a new RNA virus that was first identified in December 2019 in the city of Wuhan, China.1 SARS-CoV-2 causes a picture of atypical pneumonia that can lead to multi-organ failure.2 The deregulation of the immune system secondary to the infection produces a picture similar to haemophagocytic lymphohistiocytosis syndrome (HLHS) syndrome).3 The different pathways of immune activation culminate in cytotoxic dysfunction whose main trigger is a "cytokine storm". Several studies have defined the importance that IL-6 inhibitors (tocilizumab) have in the treatment of SARS-CoV-2 infection.4 However, the indication for treatment with IL-1 inhibitors (anakinra) has not been clearly established.
We present the case of a 51-year-old patient with bilateral pneumonia secondary to SARS-CoV-2 infection refractory to treatment with tocilizumab who showed improvement after treatment with anakinra.
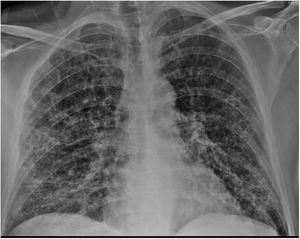
Clinical caseA 51-year-old male who attended the emergency department with a fever (>38 °C) and dyspnoea of one week's duration. The patient had a history of COPD, liver cirrhosis of unrelated origin and adenocarcinoma of the rectum (pT4N1M0). The presence of generalized hypoventilation with fine bibasal crackles was noteworthy in the physical examination. Chest x-ray (Fig. 1) showed bilateral infiltrations with a ground glass pattern. Blood analysis showed the values described in Table 1. Blood and urine cultures were negative. SARS-CoV-2 virus was detected by polymerase chain reaction in pharyngeal exudate, which was positive.
Patient's analytical values during hospital admission.
| Analytical values | Values on admission | Values at start of anakinra | 48 h after starting anakinra | Values on discharge | Range of values |
|---|---|---|---|---|---|
| Leukocytes (/μL) | 9.81 | 4.42 | 8.11 | 6.81 | (4.50−10.80) |
| Lymphocytes (/μL) | .71 | .87 | 5.60 | 1.28 | (1.20−6.50) |
| Fibrinogen (mg/dL) | 720 | – | 436 | 248 | (130−400) |
| D-Dimer (μg/mL) | .8 | 1.9 | 1.3 | 1.1 | (.0−.5) |
| Ferritin (ng/mL) | 477 | 406 | 322 | 502 | (30−400) |
| C reactive protein (mg/dL) | 19.68 | 10.01 | 1.60 | ≤.1 | (.00−.50) |
| Procalcitonin (ng/mL) | .42 | .23 | – | – | (.00−.50) |
| IL-6 (pg/mL) | – | >1000 | – | – | (.00-3.40) |
-: not available.
On diagnosis of bilateral pneumonia secondary to SARS-CoV-2 infection, the patient was admitted to hospital and treatment was initiated with broad-spectrum antibiotics (ceftriaxone, azithromycin and later escalated to piperacillin-tazobactam), hydroxychloroquine (HCQ) and lopinavir/ritonavir (LPV/r). In view of the need for respiratory support, treatment with tocilizumab was initiated (8 mg/kg every 12 h, 2 subcutaneous doses). Given the absence of respiratory and analytical improvement (Table 1) 48 h after administration of tocilizumab, it was decided to administer anakinra (100 mg single total dose, subcutaneous). Subsequently, the patient made good clinical progress, ventilatory support was discontinued and he was discharged from the hospital 14 days after admission.
DiscussionThe "cytokine storm" secondary to SARS-CoV-2 infection determines severe COVID-19 disease. The excessive activation of the immune system produces a picture similar to sHLH.3 The use of anti-IL-6 antibodies in the treatment of SARS-CoV-2 infection is currently under study, being one of the current pillars of COVID-19 disease treatment. In the study by Le et al.,5 tocilizumab demonstrated clinical response after one or two doses of the drug in 69% of patients with cytokine activation syndrome. The use of inhibitory molecules of other interleukins is currently under study, anakinra being the most studied anti-interleukin molecule after tocilizumab in the treatment of COVID-19.6
In the case of our patient, treatment with tocilizumab did not provide clinical or analytical improvement, while the use of anakinra allowed clear improvement at 48 h. Although a possible benefit due to the late effect of tocilizumab cannot be ruled out. Although a single dose of anakinra was administered to our patient, there are studies such as that of Monteagudo et al., which show the efficacy of this drug in continuous infusion at doses greater than 2400 mg/day for the treatment of macrophage activation syndrome (MAS).7
In conclusion, specific IL-1 blockade could be an effective alternative in the management of patients with SARS-CoV-2 infection who have not benefited from other treatments.
Conflict of interestsThe authors have no conflict of interests to declare. JJC-H: Consulting or Advisory Role: MSD Oncology, Bristol-Myers Squibb, Merck Speakers’ Bureau: MSD Oncology, Bristol-Myers Squibb, Merck, Roche, Janssen Oncology, AstraZeneca Travel, Accommodation, Expenses: MSD Oncology.
LF-P and AO-H contributed equally.
Please cite this article as: Figuero-Pérez L, Olivares-Hernández A, Escala-Cornejo RA, Terán-Brage E, López-Gutiérrez Á, Cruz-Hernández JJ. Anakinra, una alternativa potencial en el tratamiento de la infección respiratoria grave por SARS-CoV-2 refractaria a tocilizumab. Reumatol Clin. 2021;17:559–561.