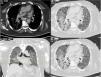
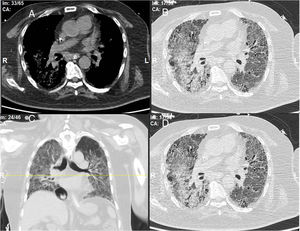
A 52-year-old male patient developed RA in March 2009 at the age of 43, with symmetric polyarthritis and active synovitis affecting hands, knees, ankles and both feet without symptoms or signs suggestive of extra-articular features. Laboratory investigations showed negative RF and positive anti-CCP antibodies, negative ANA, negative anti-dsDNA antibodies; the X-rays of both hands showed typical erosive changes in RA and fulfilled the new ACR/EULAR (2010) criteria of RA. The patient achieved remission on a combination of DMARDs. He did well until January 2017 when he developed acute onset of progressive chest pain, dyspnea, and acute respiratory failure. High-resolution CT of the lung showed extensive areas of ground glass veiling, and interstitial subpleural infiltrates were found consistent with aggressive interstitial lung disease (ILD). Autoantibodies against extractable nuclear antigens were screened and showed positive results for anti-RO and anti-Jo1 autoantibodies. The positive anti-Jo1was an expression of anti-synthetase syndrome complicating the RA course and explained the rapidly aggressive course of ILD.
Un paciente de 52 años de edad desarrolló artritis reumatoide (AR) en marzo de 2009 a la edad de 43 años, con poliartritis simétrica y sinovitis activa que afecta manos, rodillas, tobillos y ambos pies, sin síntomas o signos sugestivos de características extraarticulares. Las investigaciones de laboratorio mostraron anticuerpos anti-CCP positivos, RF negativo, ANA negativo, anticuerpos anti-dsDNA negativos; los rayos X de ambas manos mostraron cambios erosivos típicos de la AR y cumplieron los nuevos criterios ACR/EULAR (2010) de AR. El paciente logró la remisión con una combinación de DMARD. Le fue bien hasta enero de 2017, cuando desarrolló una aparición aguda de dolor de pecho progresivo y disnea, e insuficiencia respiratoria aguda. La TC de pulmón de alta resolución mostró áreas extensas de velado de vidrio esmerilado y se encontraron infiltrados subpleurales intersticiales consistentes con enfermedad pulmonar intersticial (EPI) agresiva. Los autoanticuerpos contra antígenos nucleares extraíbles se cribaron y mostraron resultados positivos para autoanticuerpos anti-RO y anti-Jo1. El anti-Jo1 positivo fue una expresión del síndrome anti-sintetasa que complica el curso de la AR y explicó el curso rápidamente agresivo de EPI.
Rheumatoid arthritis (RA) is a chronic autoimmune disorder that primarily affects synovial lined joints. The new classification criteria redefine the current paradigm of RA by focusing on features at earlier stages of disease that are associated with persistent and/or erosive disease, rather than defining the disease by its late-stage features.1 Antisynthetase syndrome (ASS) is an uncommon multisystem connective tissue disease characterized by the presence of circulating anti-aminoacyl t-RNA synthetase antibodies and clinical features of interstitial lung disease (ILD), often with inflammatory myositis and polyarthritis. Other clinical features include fever, mechanic's hand, and Raynaud's phenomenon. There is a higher prevalence and increased severity of ILD in patients with ASS, as compared with dermatomyositis (DM) and polymyositis, with which it may overlap phenotypically.2 Atypical presentations may include hand calcinosis and/or absent muscle involvement.3,4 Only a few case reports described the rare overlap between RA and ASS,5–7 the clinical presentations, immunological investigations, and disease courses of the previous case reports are detailed in Table 1. Anticitrullinated peptide/protein antibodies (ACPA)-positive ASS patients were first identified among a French multicenter registry of patients with ASS,8 and ACPA-positive ASS patients may show an overlapping features of RA-ASS syndrome and may be at high risk of developing refractory erosive arthritis.8,9
Demographic, clinical characteristics and immunological profile of the previous case reports with RA-ASS overlap syndrome and the current case report.
| Case 16 | Case 27 | Case 35 | Case 45 | Case 55 | Present case | |
|---|---|---|---|---|---|---|
| Age (years) | 63 | 56 | 70 | 52 | 60 | 52 |
| Sex | Female | Female | Female | Male | Female | Male |
| Polyarthritis | Positive | Positive | Positive | Positive | Positive | Positive |
| Myositis | Negative | Positive | Positive | Positive | Positive | Negative |
| Mechanic's hands | Negative | Positive | Positive | ND | Negative | Negative |
| Raynaud's | Negative | Negative | Negative | Positive | Negative | Negative |
| Bone erosions | Positive | Positive | Negative | Negative | Positive | Positive |
| ILD | Positive | Positive | Positive | Positive | Positive | Positive |
| CK | Normal | Elevated | Elevated | Elevated | Elevated | Normal |
| RF | Positive | Positive | Negative | Negative | Positive | Positive |
| Anti-CCP | Positive | Positive | Positive | Positive | ND | Positive |
| ANA | Positive | Positive | ND | ND | ND | Positive |
| Anti-Ro/SSA | Positive | Negative | ND | Positive | Positive | Positive |
| Anti-Jo-1 | Positive | Positive | Positive | Positive | Positive | Positive |
| Anti-PL-12 | Positive | Negative | ND | ND | ND | Negative |
| MTX | Positive | Positive | Positive | Positive | Positive | Positive |
| HCQ | Positive | Negative | Negative | Positive | Positive | Positive |
| Leflunomide | Positive | Negative | Negative | Negative | Negative | Positive |
| AZA | Negative | Negative | Negative | Negative | Negative | Positive |
| Prednisone | Positive | Positive | Positive | Positive | Positive | Positive |
| RTX | Negative | Positive | Positive | Positive | Negative | Negative |
| CTX | Positive | Negative | Negative | Negative | Positive | Negative |
ND: not done or not described; ILD: interstitial lung disease; RTX: rituximab; CTX: cyclophosphamide; RA: rheumatoid arthritis; HCQ: hydroxychloroquine; ASS: antisynthetase syndrome.
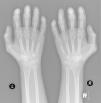
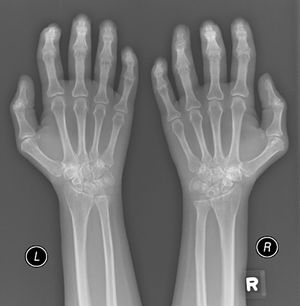
A 52-year-old male patient developed RA in March 2009 at the age of 43, with symmetric polyarthritis and active synovitis affecting hands, knees, ankles, and both feet without symptoms or signs suggestive of extra-articular features. Laboratory investigations in 2009 showed elevated markers of inflammation, negative rheumatoid factor and positive ant-CCP antibodies, negative ANA, negative anti-dsDNA antibodies; the plain X-rays of both hands showed typical erosive changes consistent with the diagnosis of RA mainly affecting the carpal bones with narrowed radio-carpal joints on both sides (Fig. 1). The patients fulfilled the ACR/EULAR (2010) classification criteria for RA.1 The patient showed much improvement on a combination therapy of disease-modifying antirheumatic drugs (DMARDs) including Methotrexate (MTX) 17.5mg/week/po, folic acid supplements, hydroxychloroquine (HCQ), and low dose of steroids (prednisolone 5mg/od/po). He had no symptoms or signs suggestive of any extra-articular features of the disease and no subcutaneous nodules, sicca symptoms or ILD. The patient showed marked improvement on the combination DMARDs and during the course of his disease he remained in remission without active synovitis or morning stiffness. He did well until January 2017 when he developed rapidly progressive shortness of breath with irrigative dry cough. On chest examination, there was bilateral infra-scapular inspiratory crepitations and high-resolution CT of the lung showed evidence of coarse reticular opacities and areas of consolidation consistent with ILD.
Plain X-ray both hands, showing cystic carpal bone erosions and decreased radio carpal joint space bilaterally, cystic erosions of the upper radius, carpo-metacarpal joints bone erosive changes and joint space narrowing of the proximal interphalangeal joints and metacarpophalangeal joints (MCP) with Juxta-articular osteoporosis.
The chest consultant considered lung affection to be related to MTX toxicity and the drug was discontinued and patient shifted to oral Azathioprine (AZA) in a dose of 150mg/day and prednisolone was increased to 15mg/day/po; he continued the same dose of HCQ. Shortly after the patient started to develop intense myalgia of both thigh muscles with no clinical evidence of motor weakness and the creatine kinase (CK) levels were within normal range. He had no Raynaud's phenomenon and no signs of gastroesophageal reflux. After 1 week, the chest symptoms worsened and the patient was admitted to the intensive care unit with severe respiratory distress. Oxygen saturation was 78% on room air and follow-up high-resolution CT (HRCT) of the lungs showed extensive areas of ground glass veiling and opacification together with interstitial subpleural infiltrates (Fig. 2). The patient was transferred to the ICU with acute respiratory failure due to exacerbation of interstitial autoimmune pneumonitis and urgent mechanical ventilation was carried out together with IV pulse steroids, IV antibiotics, and other supportive measures. During the ICU admission, the patient improved clinically and the mechanical ventilation could be discontinued; after 1 month, he was discharged on long-term oxygen therapy (2L/min), high-dose oral steroids (40mg/day/po), and oral AZA (150mg/day).
DiscussionIn this report, we described a case with longstanding RA and the rare association with ASS, which was previously reported in only a few case reports.5–7 Our patient had positive anti-CCP antibodies and on plain X-rays typical erosive changes consistent with the diagnosis of RA fulfilling the new ACR and ACR/EULAR (2010) classification criteria for RA.1 Anti-CCP antibodies are highly specific for RA but may also be found in some patients with other systemic autoimmune diseases. In Juvenile idiopathic arthritis (JIA), anti-CCP antibodies were prevalent among JIA patients with polyarticular disease pattern compared to other disease patterns and positively correlated with erosive changes10; moreover, in palindromic rheumatism early hand joint involvement and positive anti-CCP at disease onset are good predictors for progression to RA after 1 year of follow up.11 Recent studies suggest that anti-CCP antibodies can predict the development of RA in patients with early undifferentiated arthritis, allowing early individualized therapeutic decisions.12 ASS is a systemic disease characterized by the association of myositis, ILD, polyarthralgia, and/or polyarthritis, has not yet been evaluated with regard to phenotype, prognosis, and response to treatment. The clinical significance of anti-CCP antibodies in patients with ASS was first identified among a French multicenter registry of patients with ASS. Anti-CCP-positive ASS patients showed an overlapping RA-ASS syndrome were at high risk of refractory erosive arthritis and might experience ASS flare when treated with antitumor necrosis factor drugs. In contrast, other biologics such as anti-CD20 mAb were effective in this context, without worsening systemic involvements notably ILD.8 Our patient had muscle pains but normal CK levels and no evidence of neither mechanic hands nor Raynaud's phenomenon. In a landmark single-center study, polyarthritis was the first manifestation in 12 out of 45 (27%) cases with ASS who presented with polyarthritis. The mean delay from polyarthritis onset to ASS diagnosis was 27 months. Pulmonary and muscle symptoms were uncommon at initial ASS diagnosis (40 and 32.5%, respectively) and occurred with a mean delay after polyarthritis onset of 41 and 21 months, respectively. Mechanic's hands and other cutaneous signs of dermatomyositis (DM) occurred in 25 and 22.5%, respectively, with a mean delay of 10 and 31 months, respectively; Raynaud's phenomenon (RP) in (32%) was the earliest non-articular manifestation with mean delay of 3 months after polyarthritis onset. The authors ended that ASS may be revealed by polyarthritis, and to decrease the delay in diagnosis of ASS, pulmonary and muscle symptoms and anti-ARS antibodies might usefully be searched for in seronegative polyarthritis patients, especially in those with RP.13 Given that these diversities in clinical manifestations of RA-ASS overlap syndrome should be taken into consideration. The current available data regarding RA-ASS overlap syndrome are still limited and mostly came from case reports,5–7 and what seems constant in our case and other previous reports which is detailed in Table 1 is the presence of ILD, symmetric polyarthritis, and positive anti-Jo-1 autoantibody, while other manifestations of ASS syndrome may not exist, e.g. mechanic's hands, RP, and inflammatory polymyositis. This emerging new clinical entity needs further and careful interpretation in larger cohorts of patients in this domain.
Currently, our group is studying autoantibodies against extractable nuclear antigens (ENAs) in a cohort of RA patients to examine the prevalence and clinical relevance of Anti-Jo1 and to examine its association with RF, anti-CCP, ANA, and other autoantibodies against other ENAs like anti- RO, anti- LA, anti-SM, and anti-U1RNP.
In conclusion, ASS is a rare systemic disease and may complicate the course of RA, and rheumatologists should be aware of this rare underrecognized entity. Further studies are needed to examine the prevalence of anti-Jo1antibdies in RA patients and to examine its clinical relevance and its association with other autoantibodies known to exist in RA like anti-RO and anti-LA and other ENA autoantibodies for better understanding and early diagnosis of this overlapping clinical syndrome.
Declaration statementAll the authors responsible for this work declare no conflict of interest.