Vasculitis of the aorta and its major branches is a very uncommon finding in systemic lupus erythematosus (SLE). We report the case of a 65-year-old woman with SLE who developed a vasculitic process affecting the aorta.
Case ReportThe patient was a 65-year-old woman, a native of the Philippines who, at the age of 18 years had had tuberculosis, which had been properly treated but left her with pleural fibrosis. At the age of 40 years, she had been diagnosed with SLE with no associated complications, which was being followed in rheumatology outpatient clinics. She came to our department with a 1-month history of asthenia, adynamia, hyporexia and weight loss related to this syndrome (5kg in 2 months). She also complained of fever of up to 38°C accompanied by sweating and occasional chills, abdominal pain, arthralgia in both carpi and painful oral ulcers, with no other associated symptoms. The physical examination disclosed abdominal pain on palpation in right upper quadrant and in left iliac fossa, with positive response to right lumbar fist percussion and pain on palpation in both carpi, but no synovitis. The results of the blood test were as follows:
Hemoglobin: 9g/dL (range: 11.8–15.3); leukocytes: 12.07×103/μL; total neutrophils: 10.45×103/μL (2.5–8.2); 86.6% (55%–75%); total lymphocytes: 0.58×103/μL, 4.8% (25%–41%); platelets: 278×103/μL (150–450); prothrombin activity: 67% (80%–120%); international normalized ratio: 1.32 (0.8–1.2); C-reactive protein: 21.4mg/dL (0–0.8); erythrocyte sedimentation rate: 33.1mm/h (0–37); γ-glutamyl transpeptidase: 66IU/L 37°C (5–36); alkaline phosphatase: 109IU/L 37°C (35–104); haptoglobin: 527mg/dL (30–200); ferritin: 1010ng/mL (13–150); transferrin: 134mg/dL (200–360); direct and indirect Coombs tests: negative; electrophoretic pattern: normal; complement C3: 96mg/dL (90–180); complement C4: 34mg/dL (10–40); total hemolytic complement (CH50): 45.2U/mL (35–60); antinuclear antibodies (ANA): positive at a titer of 1/160 (<1/40) with a homogeneous pattern; anti-double-stranded DNA antibodies: 707IU/mL (0.1–300); antihistone antibodies: 0.44; Crithidia DNA: strongly positive; anti-Smith <1.0 (<1.0); antiribonucleoprotein (RNP) antibodies <1.0 (<1.0); antineutrophil cytoplasmic antibodies (ANCA): negative (<1/2); antineutrophil myeloperoxidase antibodies: 0.5U/mL (0–9); IgG anti-β2-glycoprotein I antibodies <9.38 SGU (0–20); IgM anti-β2-glycoprotein I antibodies <9.38 SMU (0–20); IgG anticardiolipin antibodies: 8.49GPL (0–20); IgM anticardiolipin antibodies: 3.20MPL (0–20); lupus anticoagulant antibodies: 49.2s (negative); anti-Treponema pallidum antibodies: negative; blood cultures: negative; Ziehl–Neelsen-stained sputum smear: negative; auramine-stained sputum smear: negative; Gram-stained sputum smear: negative; mycobacterium culture: negative; urinary sediment: 10–20leukocytes/field×400 (0–5); no evidence of bacteria.
Chest radiography revealed hilar enlargement, predominantly on the left side. There were no changes on abdominal radiography. The electrocardiogram showed signs of early repolarization in anterior precordial leads (V2–V4). Echocardiographic findings included a left ventricular ejection fraction of 60%, with aortic root in the upper limit of normal dimensions, and grade 1 mitral and tricuspid regurgitation with no signs of pulmonary hypertension.
Abdominal computed tomography (CT) revealed diffuse mild wall thickening affecting the entire length of the abdominal aorta and bilateral aortoiliac segment, as well as the superior mesenteric artery and common iliac arteries.
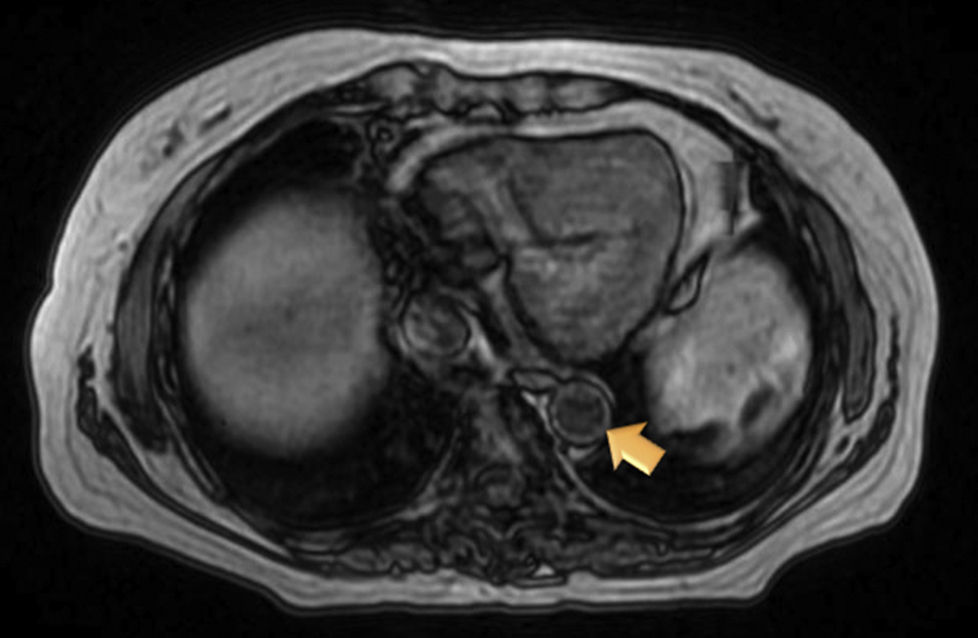
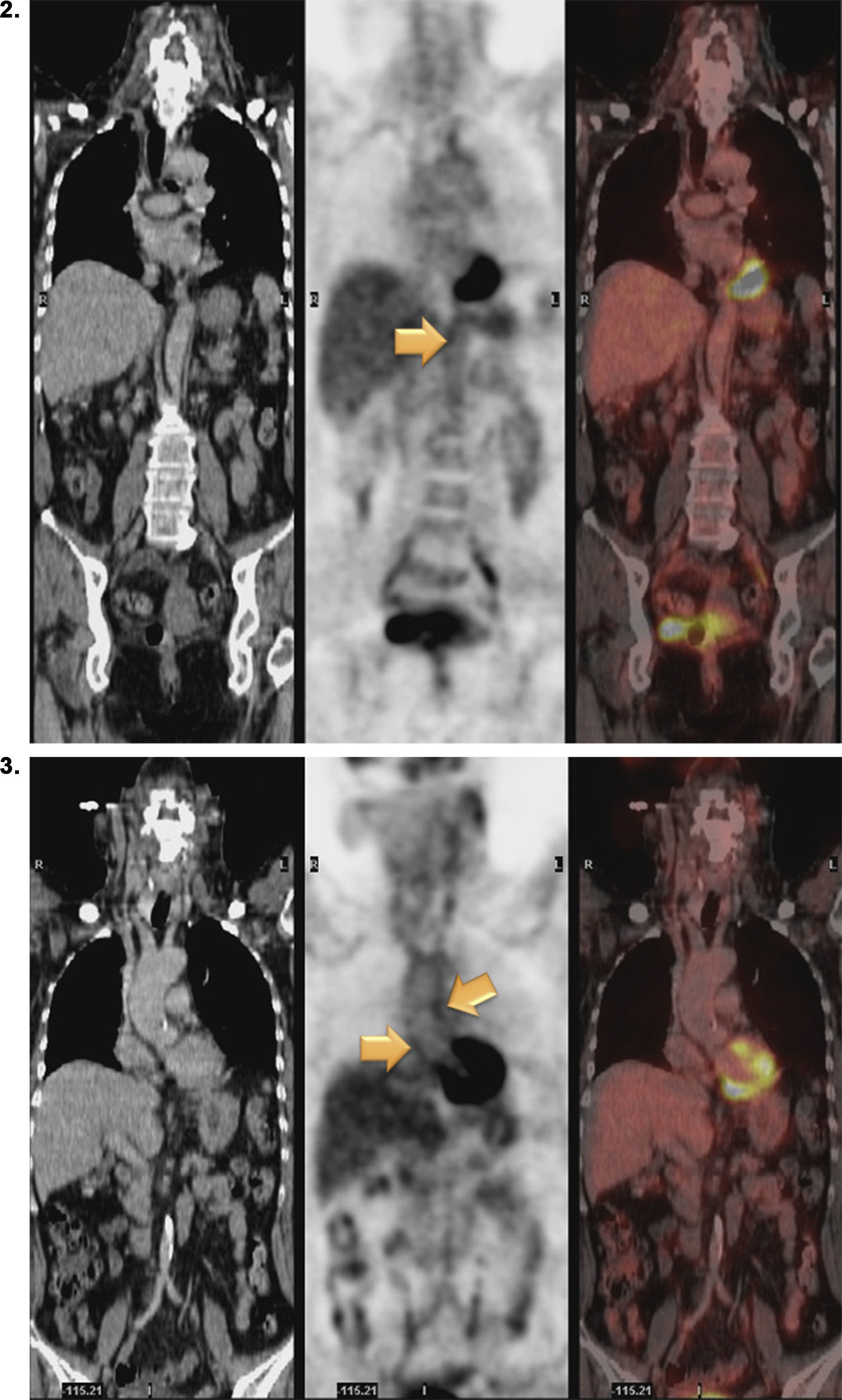
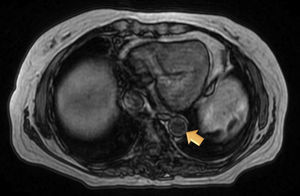
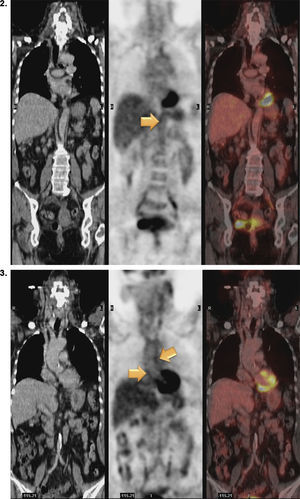
Magnetic resonance (MR) angiography of thoracic aorta and the upper portion of abdominal aorta (Fig. 1) showed a somewhat thickened aortic wall, reaching a thickness of approximately 1.7mm in prerenal aorta, with atheromatosis in thoracoabdominal aorta (thoracic CT disclosed significant calcification in thoracoabdominal aortic wall). These 2 examinations indicated a possible diagnosis of arteritis, predominantly aortitis, and constitutional symptoms in a patient with SLE. To rule out other associated processes, the patient underwent positron emission tomography (PET)/CT with 18F-fluorodeoxyglucose (FDG) (Figs. 2 and 3), which showed abnormal FDG uptake along the wall of thoracic and abdominal artery. The aortic arch, descending thoracic aorta and pulmonary artery were found to be involved. In abdomen, there was an increase in FDG uptake along the entire length of the abdominal aorta, which affected superior mesenteric artery (SMA) and extended to the common iliac bifurcation. It was associated with foci of calcified atheromatosis.
Positron emission tomography/computed tomography images with 18F-fluorodeoxyglucose (FDG) showing abnormal FDG uptake along the wall of thoracic and abdominal artery. Involvement of aortic arch, descending thoracic aorta and pulmonary artery can be seen. Increased FDG uptake is observed along the entire length of abdominal aorta, affecting superior mesenteric artery and extending to the common iliac bifurcation. Foci of calcified atheromatosis are present.
With a probable diagnosis of aortitis associated with moderate SLE and concordant metabolic activity, as well as a previous suspicion based on CT, treatment was begun with methylprednisolone at a dose of 1mg/kg during the hospital stay and later continued with mycophenolic acid (180mg/1tablet/12h). The patient's clinical signs and symptoms resolved and, at the time of writing, she was asymptomatic.
DiscussionAortitis is the inflammation of the aortic wall, with or without disruption of elastic fibers, necrosis or fibrosis, that can affect one or more layers; it is caused by a number of mechanisms.1 The incidence is around 1% to 22%, according to different series.1,2 A variety of disorders can affect the aorta: infectious diseases like syphilis, staphylococci, tuberculosis or human immunodeficiency virus, vasculitides such as giant cell or Takayasu's arteritis, connective tissue diseases like SLE and rheumatoid arthritis, or idiopathic conditions.3,4 From the clinical perspective, aortic vasculitis is nonspecific: general symptoms with fever, weight loss, general weakness, typical abdominal, back or chest pain; pain associated with aortic dissection or the vagus nerve; recurrent nonspecific pain; clinical signs of valve regurgitation; embolic phenomena; coronary ischemic or abdominal symptoms; or limb claudication.5,6
The diagnosis is based on a strong suspicion of vasculitis, imaging techniques including CT, CT angiography, MR angiography and 18F-FDG-PET, and a histopathological study.3,4,6,7
Vasculitis of the aorta and its major branches is very uncommon in SLE, and the presence of aortitis with immune complex deposition has been reported only occasionally.12 This aortitis has been associated with aortic dissections, thrombi and aneurysms.3 The development of these conditions could be related to the atherosclerotic process, as prolonged corticosteroid use plays a role in the formation of atherosclerotic plaques.3,5,13
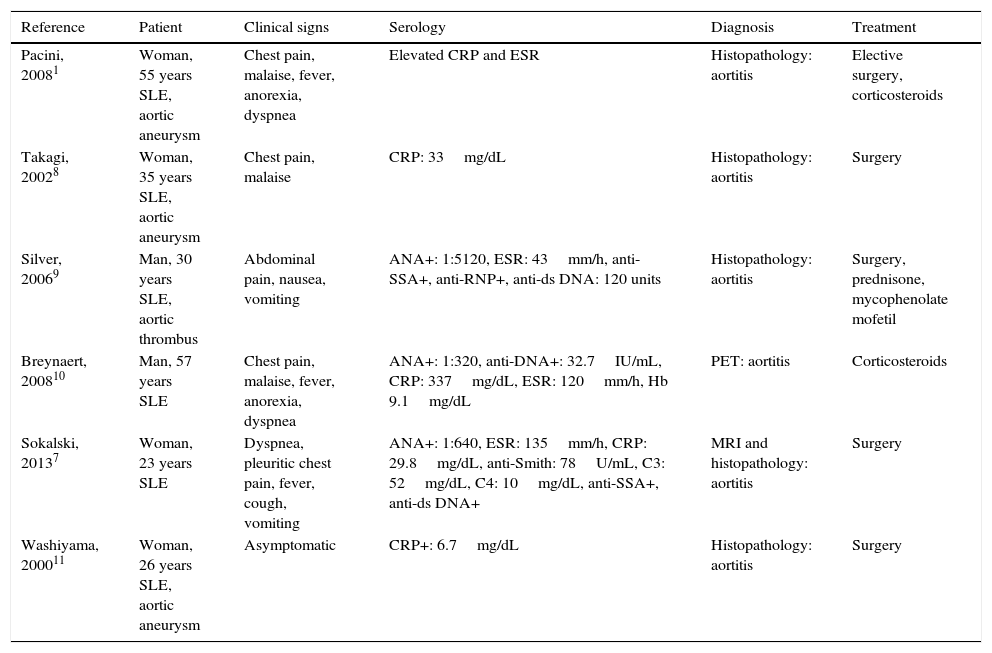
In view of the above findings, we consider the report of this case to be of interest as it highlights the need for rheumatologists to suspect a process affecting abdominal vessels when a patient presents with lupus, fever, high autoantibody titers, elevated acute phase reactants and abdominal pain. In these cases, imaging studies should be indispensable. Table 1 shows the most relevant cases of aortic involvement (vasculitis and/or aneurysm) reported in the literature.
Major Cases of Vascular Inflammation of the Aorta in Patients With Systemic Lupus Erythematosus.
| Reference | Patient | Clinical signs | Serology | Diagnosis | Treatment |
|---|---|---|---|---|---|
| Pacini, 20081 | Woman, 55 years SLE, aortic aneurysm | Chest pain, malaise, fever, anorexia, dyspnea | Elevated CRP and ESR | Histopathology: aortitis | Elective surgery, corticosteroids |
| Takagi, 20028 | Woman, 35 years SLE, aortic aneurysm | Chest pain, malaise | CRP: 33mg/dL | Histopathology: aortitis | Surgery |
| Silver, 20069 | Man, 30 years SLE, aortic thrombus | Abdominal pain, nausea, vomiting | ANA+: 1:5120, ESR: 43mm/h, anti-SSA+, anti-RNP+, anti-ds DNA: 120 units | Histopathology: aortitis | Surgery, prednisone, mycophenolate mofetil |
| Breynaert, 200810 | Man, 57 years SLE | Chest pain, malaise, fever, anorexia, dyspnea | ANA+: 1:320, anti-DNA+: 32.7IU/mL, CRP: 337mg/dL, ESR: 120mm/h, Hb 9.1mg/dL | PET: aortitis | Corticosteroids |
| Sokalski, 20137 | Woman, 23 years SLE | Dyspnea, pleuritic chest pain, fever, cough, vomiting | ANA+: 1:640, ESR: 135mm/h, CRP: 29.8mg/dL, anti-Smith: 78U/mL, C3: 52mg/dL, C4: 10mg/dL, anti-SSA+, anti-ds DNA+ | MRI and histopathology: aortitis | Surgery |
| Washiyama, 200011 | Woman, 26 years SLE, aortic aneurysm | Asymptomatic | CRP+: 6.7mg/dL | Histopathology: aortitis | Surgery |
ANA, antinuclear antibodies; CRP, C-reactive protein; ds, double stranded; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; MRI, magnetic resonance imaging; PET, positron emission tomography; RNP, ribonucleoprotein; SLE, systemic lupus erythematosus.
The standard treatment is based on the administration of high-dose glucocorticoids alone or in association with immunosuppressive agents like cyclosporine or mycophenolate mofetil. Alternative treatments in the case of refractory disease include the intravenous administration of immunoglobulins, rituximab or plasmapheresis.12 At the time of writing, the patient was asymptomatic and stable, without corticosteroids in their usual regimen, and receiving mycophenolic acid at a dose of 360mg/12h.
The major limitation of this study is the fact that it was not possible to confirm the diagnosis histologically because of the difficulty involved in the technique and the site of the vasculitis. One finding that indicates that the vasculitis was associated with SLE was its improvement after treatment with immunosuppressive therapy associated with high doses of corticosteroids. (The outcome would have been different if, for example, the etiology had been infectious.)
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Pérez Ruiz J, Salman-Monte TC, Pros-Simón A, Mestre-Fusco A, Carbonell i Abellò J. Vasculitis aórtica en un paciente con lupus eritematoso sistémico. Reumatol Clin. 2016;12:169–172.