We report the case of a 65-year-old woman with psoriatic arthritis who developed aortitis secondary to giant cell arteritis. She presented with a 2-month history of dry cough, fever and fatigue. There was no evidence of tumor or infectious processes. Abdominal computed tomographic and computed tomography coronary angiographic findings were suggestive of aortitis. Histological study of a temporal artery biopsy confirmed temporal arteritis. We also review the available literature on this uncommon condition.
Presentamos el caso de una mujer de 65 años seguida por artritis psoriásica, que desarrolló una aortitis por arteritis de células gigantes, con un comienzo de tos seca, fiebre y astenia de 2 meses de evolución. Se realizó estudio descartando procesos tumorales e infecciosos y evidenciándose en la TAC abdominal y la angio-TAC datos indicativos de aortitis. Se realizó biopsia de arteria temporal con confirmación histológica de arteritis de células gigantes. Realizamos una revisión de la información disponible sobre esta infrecuente asociación.
Giant cell arteritis (GCA) is a systemic vasculitis involving medium and large vessels, with an annual incidence of 12–17 cases/100,000 population. It occurs more frequently in women and generally affects individuals over 50 years of age. The branches of the external carotid artery are those most commonly involved, although aortitis can develop in a certain subtype of patient. The involvement of the aorta is probably underestimated: subclinical aortitis has been detected in 20%–65% of the patients at diagnosis.1 It should be considered in those with atypical presentations of GCA, with data indicating systemic inflammation or a relapse during treatment,2 although it is asymptomatic in the majority of patients, or is manifested by means of systemic signs like fever or constitutional symptoms,3 as observed in our patient.
On the other hand, psoriatic arthritis (PsA) has a prevalence of 0.5%, affecting between 5% and 20% of patients with psoriasis.4 In a retrospective study, Makredes et al. found that PsA had a higher prevalence ratio associated with GCA.5 To date, there have been reports of 2 patients with PsA who developed GCA.6,7
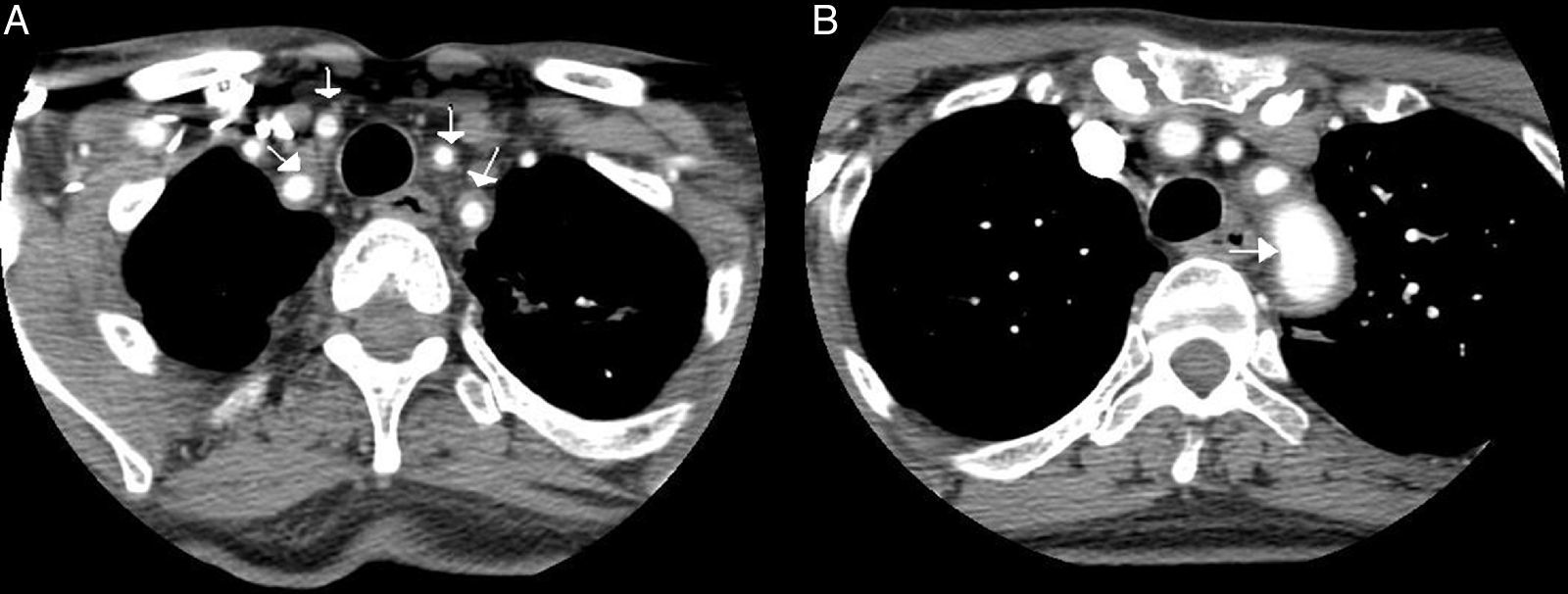
Case ReportOur patient was a 65-year-old woman with a history of bronchial asthma, with bronchiectasis, and PsA with mixed erosive involvement; she was negative for HLA-B27. She had received methotrexate and leflunomide from 2011 until 2013, when they were interrupted because of remission. She was presented with a 2-month history of fever and weakness, but did not report headaches, vision disorders or chest, abdominal or limb pain. Physical examination revealed only a difference in arterial blood pressure of 20mmHg and that some pulse rates were lower in left limbs. The temporal arteries were not tender on palpation, and the pulse rate was normal and symmetric. Laboratory tests showed a hemoglobin concentration of 9.9g/dL (normal: 12.0–15.0), erythrocyte sedimentation rate of 102mm/h (0–20), C-reactive protein 140.90mg/L (<5mg/L) and iron 11μg/dL (35.0–145.0). The remaining parameters were normal (thyroid-stimulating hormone, creatine phosphokinase, Mantoux test, tumor markers, protein profile, serological tests, immunological tests, urinary sediment and cultures). Chest radiography, echocardiogram, gastroscopy with duodenal biopsy and colonoscopy were normal. Chest and abdominal computed tomography (CT) showed stable bronchiectasis and thickening of the abdominal aortic wall, indicative of aortitis. Computed tomography angiography (CTA) (Fig. 1) also showed evidence of concentric inflammatory thickening of the supra-aortic trunks and aortic arch. Temporal artery biopsy was performed with the histological confirmation of GCA. Treatment was begun with corticoids at a tapering dose starting with 2mg/kg/day and methotrexate at 15mg/weekly, and the symptoms disappeared.
Computed tomography angiography: the presence of a concentric inflammatory thickening/vasculitis of all the supra-aortic branches (A) and of the aortic arch (B) is confirmed (arrows). The greatest involvement appears to be that observed in left subclavian artery, and there is no evidence of significant stenosis at any point. In the carotid territory, only the common carotid artery is affected, there being no extension to internal carotid artery.
We present the case of a patient with PsA in remission who developed GCA. Reviewing the literature, we found only 2 other examples; in neither of them was aortic involvement associated with GCA. In 1989, Clementz et al.6 reported the case of a 63-year-old woman with PsA who developed GCA associated with pericarditis and pancreatic insufficiency. Corli et al.7 described that of a 62-year-old man with PsA and ulcerative colitis, treated with adalimumab, who developed GCA. They comment on whether anti-tumor necrosis factor (TNF) therapy could act as an inducer; they ultimately conclude that this association cannot be substantiated and appears to be merely a coincidence. Makredes et al.5 observed that PsA is more frequently associated with the development of other autoimmune diseases, such as inflammatory bowel disease (1.8 [1.3–2.5]), pulmonary fibrosis (1.9 [1.2–3.0]) and, above all, GCA (4.8 [1.5–15.7]). This may be due to the fact that certain autoimmune diseases share the same pathogenic pathways, like TNF, which is implicated in the pathogenesis of PsA and GCA. Elkayam et al.8 reported 5 cases of men diagnosed with ankylosing spondylitis who developed polymyalgia rheumatica (PMR). Another study showed a higher prevalence of spondyloarthritis (SpA) in patients newly diagnosed with large vessel vasculitis, specifically in 4 of the 15 patients included (6 with GCA and 9 with PMR). In addition, these patients with concomitant SpA were younger and had higher baseline C-reactive protein levels. The major limitation of that study is the small size of the sample, a circumstance that means that the authors were not able to draw significant conclusions.9 Recent pathophysiological findings suggest that vasculitis and SpA share a common inflammatory process: the expression of interleukin (IL) 22 was demonstrated in mouse aortic root, as well as in the initial SpA enthesitis, after exposure to IL-23.10 However, despite the findings of these studies, it is not clear whether there is an association between GCA and chronic SpA, or if they are simply 2 concurrent autoimmune diseases.
On the other hand, aortitis secondary to GCA is a common subclinical entity.1 In most cases, this inflammatory aortitis is asymptomatic or is only manifested by systemic signs like fever and other constitutional symptoms,3 as in our patient. For the early diagnosis of aortitis due to GCA, both positron emission tomography (PET) and magnetic resonance imaging are effective.2 The former using 18F-fluorodeoxyglucose as a tracer shows an increased metabolic uptake in aorta in approximately 50% of the patients with GCA, enabling direct visualization of the extension of the vascular inflammation. Aortic CT scans provide images of aneurysmal dilatation, ectasia and focal or concentric parietal thickening. Occasionally, abdominal ultrasound makes it possible to visualize the thickening of the vascular wall, with a hypoechoic “halo” around the abdominal aorta, which is indicative of aortitis.1 Some authors propose performing screening for aortic complications based on chest radiography and abdominal ultrasound, possibly completed with CTA at the time of diagnosis, in order to prevent serious complications, like aneurysm rupture or aortic dissection.3
The differential diagnosis of inflammatory diseases of the aorta comprises a wide spectrum of etiologies from infectious processes to noninfectious disorders like vasculitis, sarcoidosis and IgG4-related lymphoplasmacytic aortitis, and even as a cardiovascular complication secondary to SpA.11 The literature includes the description of 7 cases of abdominal aortitis due to SpA.12,13
In terms of management, the involvement of the aorta and other great vessels in GCA does not change the treatment strategy, which is based initially on corticosteroid therapy.2
ConclusionsThe case presented here demonstrates the importance of screening for aortic involvement in GCA due to its prognostic and therapeutic implications. Finally, the coexistence between PsA and GCA seems to occur infrequently. At this time, the question as to whether there is an association, or if they simply represent 2 concurrent autoimmune diseases, will need to be evaluated in future studies.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: García-Cezón de la Cruz MP, Almodóvar R, García Pérez J, Dhimes PF, Zarco P. Aortitis por arteritis de células gigantes y artritis psoriásica: una asociación infrecuente. Reumatol Clin. 2017;13:230–232.