A 45-year-old man presented to the emergency room with a 15-day history of myalgia of insidious onset and progressive course. It began with symmetrical weakness and pain in feet and ankles that extended upward to the knees. Later, this progressed to paraparesis with creatine phosphokinase level of 44270U/L and respiratory failure that required mechanical ventilation. Electromyography and muscle biopsy of quadriceps were performed. The patient responded to corticotherapy in pulses and supporting management. The presentation of ascending paresis suggested the diagnosis of Guillain–Barré syndrome. However, the degree of muscle involvement with rhabdomyolysis explains the neurological damage by itself. The biopsy revealed pathological criteria for necrotizing autoimmune myopathy (NAM), as well as other clinical and laboratory evidence. The patient's disease progressed and reached the criteria for systemic lupus erythematosus (SLE). To our best knowledge, this is the first report of the NAM and SLE association.
El paciente, un hombre de 45 años de edad, ingresó a Emergencias debido a un padecimiento actual que inició 15 días antes de forma insidiosa y curso progresivo. Comenzó con debilidad simétrica y dolor en los pies y los tobillos, que se extendió hacia arriba hasta las rodillas. Más tarde, el dolor progresó a paraparesia, mostrando niveles de creatincinasa de 44.270U/L e insuficiencia respiratoria que requirió ventilación mecánica. Se llevaron a cabo una electromiografía y un biopsia muscular del cuádriceps. El paciente respondió a la corticoterapia en pulsos y a manejo de soporte. La presentación de parálisis ascendente sugirió el diagnóstico de síndrome de Guillain-Barré; sin embargo, el grado de afectación de los músculos con rabdomiólisis explicó el daño neurológico por sí mismo. La biopsia reveló criterios patológicos para miopatía necrosante autoinmune (MNA), así como otros datos clínicos y de laboratorio. Además, reunió criterios para clasificarse como lupus eritematoso sistémico (LES). De acuerdo con la literatura revisada, este es el primer reporte de la asociación entre MNA y LES.
Inflammatory myopathies are a heterogeneous group of rare acquired acute, subacute, chronic muscle diseases which have in common the presence of proximal muscle weakness associated with inflammation on muscle biopsy.1 Because these diseases occur as the largest group of potentially treatable acquired myopathies, early recognition is clinically important.2
The inflammatory myopathies include classical polymyositis, dermatomyositis, sporadic inclusion body myositis, overlap syndrome and nonspecific myositis.3 The incidence of this group of diseases is 1 per 100000 population.4
Necrotizing myopathies, a subgroup of inflammatory myopathies,5 with little inflammatory component, were first reported by Emslie-Smith and Engel.6 In 2004, the Muscle Study Group proposed a separate classification for an entity that is recognized with increasing frequency, autoimmune necrotizing myopathy (ANM), in accordance with its immunopathological, histological and clinical presentation.3 Clinically, ANM presents with predominantly proximal symmetrical weakness of upper and lower limbs, with an acute or subacute onset, coupled with high levels of creatine kinase (CK) and myopathic findings on electromyography. On pathologic examination, no prominent inflammatory infiltrate is seen, with macrophages rather than T cells as effector cells.1 In retrospect, it has been observed that a high proportion of inflammatory myopathies described as polymyositis were ANM.7
When reviewing the literature, there are review articles and case reports associated with autoimmune diseases, drug toxicity and malignancy.3 However, no studies have been found regarding prevalence or incidence. The objective of this case report is to present a potentially treatable acute presentation so that it becomes a part of the initial differential diagnosis in this clinical entity.
Description of the CaseThe patient is a 45-year-old male from Lima whose job as a street vendor involved intense walking; he presented to the emergency room due to myalgia, muscle weakness in the distal lower limbs, and numbness of feet and hands, in addition to fever of 40°C for the first 3 days, which had lasted 15 days. His knees were affected in the following days, until 10 days before admission when the discomfort intensified. During that time, he went to the health center where he was prescribed NSAIDs and muscle relaxants. On admission, the pain and weakness were predominantly proximal in both upper and lower limbs, making him unable to stand.
As a medical history, he had an allergy to penicillins, had typhoid fever at age 14, kidney stones at age 15 and dengue infection at age 32. He was diagnosed with hypertension and received irregular treatment with captopril 25mg; he used steroids weekly for 6 weeks prior to admission. He had positive tobacco use and sporadic alcohol consumption. One of his maternal aunts died of cervical cancer.
He presented constipation for 6 days before admission. On physical examination, vital signs showed a heart rate of 100 beats per minute, respiratory rate of 28 breaths per minute, blood pressure 90/60mmHg and temperature 37.2°C. The patient was diaphoretic and had tenderness of the legs. Sensitivity was preserved. He had reduced passive muscle tone with no resistance to movement upon gait. Proximal and distal active motility and strength were 2/5 in the upper limbs and 3/5 in the lower limbs. On examination of the nervous system, the biceps, patellar and Achilles tendon reflexes were ++++ symmetrically and the evaluation of the cranial nerves showed no abnormalities.
Laboratory tests showed leukocytosis, lymphopenia, and CK 44270U/L (Table 1). Urinalysis presented more than 100 erythrocytes and 10–20 leukocytes per field, protein ++/+++ and occult blood +++/+++. Viral or bacterial infection (HIV serology, syphilis, HTLV-I, HBV, HCV, cytomegalovirus, Epstein–Barr virus, Cryptoccocus sp. and pneumonia) was ruled out. Toxicology ruled out the association of symptoms with toxic etiology.
Key Laboratory Variables.
| At Hospitalization | Hospitalized | Discharge | |
| Hemoglobin (g/dL) | 16.3 | 10.3 | 13.1 |
| Hematocrit | 46.8% | 30.7% | 39.9 |
| Leukocytes (/cm3) | 28900 | 20000 | 15900 |
| Neutrophils | 6% | 2% | 4% |
| Lymphocytes | 2% | 2% | 10% |
| Eosinophils | 4% | 1% | 0% |
| Platelets (/cm3) | 272000 | 92000 | 333000 |
| Albumin (g/dL) | 2.11 | 1.64 | 3.92 |
| Globulins (g/dL) | 2.12 | 2.44 | 2.53 |
| INR | 1.2 | 1.52 | |
| Creatine kinase (U/L) | 44270 | 11840 | 128 |
| CK-MB (U/L) | 820 (<6%) | 202 (<6%) | 57 (>6%) |
| LDH (U/L) | 6620 | 4040 | 561 |
| Urea (mg/dL) | 146 | 216 | 37 |
| Creatinine (mg/dL) | 0.9 | 0.84 | 0.23 |
CK-MB: creatine kinase MB fraction; INR: international normalized ratio; LDH: lactate dehydrogenase.
Electromyography reported a neurogenic–myogenic mixed pattern, no abnormal spontaneous activity of the denervation and fibrillation type. Probable acute inflammatory predominantly demyelinating polyradiculitis was considered after cerebrospinal fluid analysis. CSF showed no albumin-cytologic dissociation, a cell count of 5, 10% polymorphonuclear, mononuclear 10%, glucose 83mg/dL and protein 26mg/dL.
Given the above, flaccid paralysis with a suspected autoimmune, toxic, metabolic or neoplastic cause, as well as acute respiratory failure associated to hypoxemia and pneumonia, rhabdomyolysis with suspected toxic or inflammatory etiology, systemic inflammatory response syndrome, compensated respiratory alkalosis, hyponatremia, hypocalcemia and hyperlactatemia were all considered.
During the progression of the disease, the respiratory condition worsened and in the following days the laboratory findings were interpreted as respiratory failure (hypoxemic and related to ventilation), with metabolic acidosis with respiratory acidosis and azotemia, and a Glasgow score of 10 leading to mechanical ventilatory support. Methylprednisolone pulse therapy 1000mg/24h intravenously, once daily for 3 days was begun for suspected autoimmune etiology.
In chronological order from the fifth to the sixteenth day of hospitalization, the patient presented positive anti-dsDNA (1/160), paranoid psychosis, with predominantly visual hallucinations, proteinuria 1000mg/dL, seizures, laminar bilateral pleural effusion seen by ultrasonography and thrombocytopenia 25000U/cc. With these two criteria to the diagnosis of systemic lupus erythematosus (SLE)8 was considered.
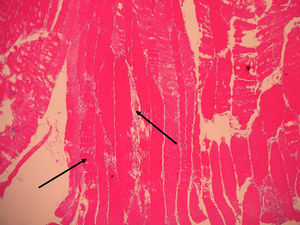
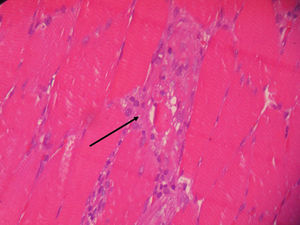
Vastus lateralis muscle biopsy of the quadriceps indicated the presence of an inflammatory infiltrate with macrophages from the destroyed muscle fibers and absence of vasculitis, findings consistent with necrotizing myopathy (Figs. 1 and 2).
The route of administration of corticosteroid therapy was changed to oral prednisone 70mg/day, in addition to antibiotics, anticoagulants, gastroprotective agents and management of the fluid and electrolyte balance. The dose of prednisone was gradually decreased. We withdrew the endotracheal intubation 3 weeks after hospitalization; the patient was discharged into the care of Internal Medicine with standard blood gas values and blood count Hb 10.6/L, hematocrit 27.8L/L, leukocytes 10,390/cm3, neutrophils 0%, 9% lymphocytes, platelets 410000, CK 3.77U/L, urea 42mg/dL and creatinine 0.4mg/dL. Urinary sediment showed 8–10 leukocytes and 2–4 erythrocytes per field, and protein and occult blood were negative. Respiratory and hematological problems improved. The prednisone dose of 60mg/day orally was decreased. The strength of upper and lower limbs remained at 2/5 and 3/5, respectively. Oncology discarded a neoplastic etiology (alpha-fetoprotein, carcinoembryonic antigen, Ca19.9, Ca72.4, PSA, and B2-microglobulin were all negative).
He was discharged with instructions to continue physical therapy and rehabilitation and prednisone 50mg/day and control by Internal Medicine. Nine months after discharge, the patient discontinued his visits to the hospital, but continues prednisone 20mg/day, calcium, vitamin D and 75 mg pregabalin for pain management, prescribed by a doctor from another health center. At the time of writing this paper, he performs physical therapy exercises at home and walks with the aid of a cane.
DiscussionThe initial analysis of the patient suggested the diagnosis of Guillain–Barré syndrome, given the clinical features of this patient, the presence of distal muscle weakness that started symmetrically in the lower limbs, ascending and the progression to respiratory failure and need for mechanical ventilation. However, feet paresthesia and hands should be evaluated with caution when considering the clinical criteria, because in the case of Guillain–Barré syndrome these usually precede weakness and do not appear concomitantly, as in this case.9 Furthermore, the presence of high fever for the first 3 days obliged us to think about other likely diagnoses and to question the diagnosis of Guillain–Barré.10 Likewise, the favorable response to corticosteroid therapy makes the initial diagnosis of Guillain–Barré syndrome even less probable and supports the possibility of autoimmune diseases.11,12
The high levels of CK and LDH were evidence of muscle destruction and, in addition to the edema of the affected limbs, made it necessary to consider the diagnosis of myositis; the rapid progression and intensity and laboratory values made us think of a disease of necrotizing nature.
The use of steroids has been reported as a possible cause of rhabdomyolysis in several publications.13,14 Intense exercise, a factor in our case because of the working hours of the patient, has also been reported as a possible trigger.15,16
The clinical and pathological findings of the case study were explained and were compatible with inflammatory autoimmune necrotizing myopathy. ANM is characterized by the absence of prominent inflammatory infiltrate and the presence of macrophages as effector cells instead of T cells; in addition, it usually does not demonstrate the immunohistochemical expression of MHC-I,1,6,17 as in polymyositis. Generally, it is clinically characterized by a subacute onset of proximal weakness and symmetrical affection, elevated CK levels and findings suggestive of myopathy on electromyography, as well as for its response to high doses of corticosteroids.2,18 However, in the case above, the onset was distal and progression proximal. ANM has been associated with connective tissue disorders, viral infections, drugs, in particular HMG-CoA reductase inhibitors or statins,19 and malignancy.3,20 Anti-SRP antibody has been described as a marker of ANM, especially related to statins, but there are no commercial kit available for its evaluation.21
The patient history ruled out the use of statins or other toxic agents associated with ANM, and laboratory tests found no positive viral antigens such as HIV, HBV or HTLV, which may also be associated3; the response to treatment and follow-up for 9 months ruled out both neoplastic and toxic etiology10 so it follows that the patient had an autoimmune disease triggered by consumption of anabolic steroids, excessive physical exercise along with sun exposure, coupled with a genetic susceptibility which was not reported in the family history.
During the course of his illness, the patient had more than 4 classification criteria for SLE, which does not exclude the diagnosis of ANM, but could eventually be considered as an overlap syndrome. No cytotoxic agents such as azathioprine, cyclophosphamide or mycophenolate mofetil were used and, although the clinical practice guidelines for SLE indicate that these agents prevent long-term recurrence, many patients respond to treatment with corticosteroids alone, as in this8,22 case. SLE may explain the neurological symptoms that initially indicated Guillain–Barré syndrome, an association reported in the literature.10,23,24 However, there was no patient albumin-cytology dissociation or some other clinical features previously discussed.
Also, the literature reports a variable association of SLE with muscle disorders that may lead to25,26 rhabdomyolysis, cases in which there is no doubt of the evidence of infection or reaction to a drug, as well as inflammatory myopathies.27–29 However, the association presented in this patient of an ANM type of inflammatory myopathy, with an unusual distal presentation, and SLE not being reported highlight the importance of this case. When faced with a similar clinical case, the diagnosis must be quickly established as the life of the patient may be compromised and multidisciplinary treatment needed.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that no experiments have been performed on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained informed consent from patients and/or subjects referred to in this article. This document is in the possession of the corresponding author.
Conflict of InterestThe authors declare no conflicts of interest.
We thank pathologist César Chian García for the pathologic descriptions of the slides of the patient. We also express our gratitude to Luis Torres Ramírez, neurologist, for the details on the neurological manifestations of the patient's case and for reviewing the manuscript.
Please cite this article as: García-Reynoso MJ, Veramendi-Espinoza LE, Ruiz-Garcia HJ. Paresia ascendente como comienzo de inusual asociación entre miopatía autoinmune necrosante y lupus eritematoso sistémico. Reumatol Clin. 2014;10:183–186.