There is sufficient evidence of the capacity of silica to induce autoimmunity in patients with some type of genetic susceptibility. There are several autoimmune diseases related to this exposure (rheumatoid arthritis, Sjögren's syndrome, sarcoidosis, and systemic sclerosis). Nodular silicosis (clinical expression of this exposure in lungs) generates apoptosis, inflammation, loss of tolerance and a respiratory burst. There is evidence that relates silica with induction of antineutrophil cytoplasmic antibodies, but, until it is better explained, the reports of systemic vasculitis secondary to silica exposure are inconclusive. We describe the case of a patient with a history of occupational exposure to silica who developed microscopic polyangiitis.
Existe suficiente evidencia de la capacidad de la sílice de inducir autoinmunidad en pacientes con algún tipo de susceptibilidad genética. Existen varias enfermedades autoinmunes relacionadas con esta exposición (artritis reumatoide, síndrome de Sjögren, sarcoidosis, esclerosis sistémica). La silicosis nodular (expresión clínica pulmonar de esta exposición) genera fenómenos de apoptosis, inflamación, pérdida de la tolerancia y explosión respiratoria. También se ha descrito la inducción de anticuerpos anticitoplasma del neutrófilo con este mineral, pero hay reportes no concluyentes de vasculitis sistémicas secundarias a la exposición a la sílice. Se describe el caso de un paciente con antecedente de exposición ocupacional a sílice que desarrolla una poliangiítis microscópica.
Silica exposure has been linked to autoimmune diseases (rheumatoid arthritis, systemic sclerosis, Sjogren's syndrome, and sarcoidosis).1 An association has been described between this mineral and the presence of ANCA but, to the best of our knowledge, there are no conclusive reports of induction of systemic vasculitis.
Clinical ObservationThe patient was a 34-year-old male who, for the past 6 years, worked as a marble installer. He had other relevant medical history. He came to the clinic after 7 days of progressive edema of the lower limbs, which progressed to anasarca, fatigue, weakness and oliguria. Review of systems: Negative. His blood pressure was 160/100mmHg and he was pale; the rest of the examination provided no relevant data. The patient developed nephritic and nephrotic syndromes. The results of paraclinical studies on admission are seen in Table 1.
The hepatic function tests, antinuclear antibodies, antibodies against core extractable antigens, anti-DNA, complement levels, antistreptolysin, hepatitis B and C, ELISA for human immunodeficiency virus and syphilis serology were negative. ANCA were positive at 1:160, with a perinuclear pattern by indirect immunofluorescence and positive myeloperoxidase antibodies by ELISA (normal values 0–20, 133 international units). We also ruled out Goodpasture syndrome (negative glomerular basement membrane antibodies) and other ANCA-associated vasculitis by clinical features and negative specific autoantibodies.
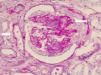
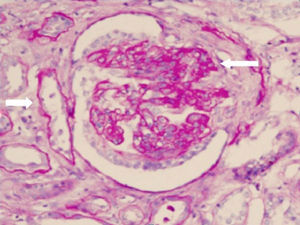
Renal biopsy revealed glomerulonephritis with global and segmental sclerosis and a collapsing aspect, glomerular hypoperfusion, podocyte hypertrophy, interstitial fibrosis and tubular atrophy of over 70%, with evidence of fibrinoid necrosis and karyorrhexis, with extracapillary proliferation (Fig. 1). Cyclophosphamide 200mg intravenously (IV) and methylprednisolone 500mg IV daily for 3 days and then prednisolone 1mg/kg/day in divided doses were started, and renal replacement therapy (intermittent hemodialysis) was indicated. The total time of hospitalization was 10 days.
The patient was readmitted 3 weeks later due to malaise, fever and hemoptysis. A chest X-ray showed evidence of reticular and diffuse alveolar interstitial infiltrates. A lung biopsy showed nodular silicosis. A new dose of cyclophosphamide and methylprednisolone, same as the above, was administered, with improvement of respiratory symptoms. No significant changes occurred with respect to the values of azotemia, urinary sediment or 24h proteinuria.
Two weeks later, the patient was readmitted for dyspnea and tachypnea. He presented hypoxemia (oxygen saturation 85%), hemoptysis, and drop in hemoglobin from baseline (8–6g/L); no clinical focus of bleeding or biochemical evidence of hemolysis was identified. There was a rapid progression to hypoxemic respiratory failure requiring ventilatory support. Bronchoalveolar lavage with the presence of 45% hemosiderophages without infection was practiced. Carbon monoxide diffusion was 125% (normal up to 90%). Three sessions of plasmapheresis were performed, but the patient was hemodynamically unstable, with high ventilatory requirements, and died.
DiscussionThe initial organ affected with silica exposure was the lung (silicosis). Necrosis and apoptosis of alveolar macrophages, production of proinflammatory cytokines (tumor necrosis factor, interleukin-1), increased cytotoxic T cell survival, decreased regulatory cells and enhanced reactive oxygen species are phenomena generated. In general, the patient had few symptoms; dyspnea should prompt a complication or another associated entity.1
This has also been linked with the production of ANCA; they may be directed against various antigens (proteinase 3, myeloperoxidase, lactoferrin and bactericidal permeability increasing protein).2,3 To the best of our knowledge, reported cases of vasculitis secondary to silica are controversial.4–7
Bartůnková et al. analyzed 86 individuals exposed to silica for at least 5 years; presence of ANCA was more frequent in those exposed (17.1%) than in controls (3.6%). The odds ratio (OR) was 5.04 (95% CI, 1.2–21.2).8
The case being reported is similar to those described in the literature: Tervaert et al. explored the relationship between silica and the development of systemic vasculitis, finding an OR for renal failure after rapidly progressive glomerulonephritis of 2.5 (95% CI, 1.37–4.60) and 6.5 for pulmonary vasculitis (95% CI, 1.4–11.6).9 Hogan et al., in a case–control study, found an increased risk of small-vessel vasculitis associated with ANCA after high exposure to silica (5 years) (OR 1.9, 95%, 1–3.5, P=.05).10 The pathophysiology is similar to that of silicosis, but the target cell is a neutrophil.
ConclusionThis case illustrates the probable association between severe microscopic polyangiitis and prolonged exposure to silica. Given the limited number of reported cases of this association, the prevalence of silicosis among those exposed and the frequency of development of autoimmune diseases remain unknown.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that no experiments have been performed on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: Vega Miranda J, Pinto Peñaranda LF, Márquez Hernández JD, Velásquez Franco CJ. Poliangiítis microscópica secundaria a exposición a sílice. Reumatol Clin. 2014;10:180–182.