Several antibodies have proven to be useful in autoimmune diseases, as markers for diagnosis, prognosis or clinical manifestations. Our objective was to evaluate the diagnosis and manifestations associated for antibodies anti-Ro52, anti-Ro60 and anti-La at a referral hospital in Spain.
MethodsWe retrospectively analyzed the antigenic specificities of the consecutive samples submitted to the Immunology Unit for antinuclear antibody screening between 2002 and 2012. We included patients with more than one positive sample for some of the autoantibodies anti-Ro52, anti-Ro60 or anti-La. We also reviewed diagnosis, clinical and laboratory features. As dependent variable we evaluated possible combinations of anti-Ro52, anti-Ro60 and anti-La.
Results322 patients, 91% females, were studied (age 44.3±15.51 years). The most frequent diagnosis was Sjögren's syndrome (40.06%) and systemic lupus erythematosus (SLE) (36.6%). The most prevalent pattern by indirect immunofluorescence was the fine speckled (69.9%). Anti-Ro52+/anti-Ro60+/anti-La+ combination was positively associated with fine speckled pattern (p: 0.001) and negatively with homogeneous (p: 0.016) and cytoplasmic pattern (p: 0.002). Isolated anti-Ro52+ was negatively associated with fine speckled pattern (p<0.001) and positively with the cytoplasmic one (p<0.001). The main positive associations with clinical symptoms were xerostomia and xerophthalmia with anti-Ro52+/anti-Ro60+/anti-La+ (p<0.001), oral ulcers with anti-Ro52+/anti-Ro60+/anti-La− (p: 0.002) and alopecia with anti-Ro52−/anti-Ro60+/anti-La− (p: 0.003). Negative associations were xerophthalmia and photosensitivity with anti-Ro52+/anti-Ro60−/anti-La− (p: 0.003). Laboratory positive associations were hypergammaglobulinemia with anti-Ro52+/anti-Ro60+/anti-La+ (p: 0.003), and hypocomplementemia with anti-Ro52−/anti-Ro60+/anti-La− (p: 0.003). Leucopenia was negatively associated with anti-Ro52+/anti-Ro60−/anti-La− (p: 0.003).
ConclusionOur study found significant relationships between clinical and laboratory manifestations with different patterns of antibodies to anti-Ro52, anti-Ro60 and anti-La. The combination of antibodies might be clinically useful due to prognostic and therapeutic implications.
Varios anticuerpos han demostrado ser útiles en enfermedades autoinmunes, como marcadores de diagnóstico, pronóstico o manifestaciones clínicas. Nuestro objetivo fue evaluar el diagnóstico y las manifestaciones asociadas a anticuerpos anti-Ro52, anti-Ro60 y anti-La en un hospital de referencia en España.
MétodosSe analizaron retrospectivamente las especificidades antigénicas de todas las muestras consecutivas solicitadas a la Unidad de Inmunología para la detección de anticuerpos antinucleares entre 2002 y 2012. Se incluyeron pacientes con más de una muestra positiva para algunos de los autoanticuerpos anti-Ro52, anti-Ro60 o anti-La, y se revisaron sus características diagnósticas, clínicas y de laboratorio. Como variable dependiente se evaluaron las combinaciones de anti-Ro52, anti-Ro60 y anti-La.
Resultados322 pacientes, 91% mujeres, fueron estudiados (edad 44.3±15.51 años). El diagnóstico más frecuente fue el síndrome de Sjögren (40.06%), y el lupus eritematoso sistémico (LES) (36.6%). El patrón por inmunofluorescencia indirecta más prevalente fue el moteado fino (69.9%). La combinación Anti-Ro52+/anti-Ro60+/anti-La+ se asoció positivamente con el patrón moteado fino (p: 0.001) y negativamente con el homogéneo (p: 0.016) y el citoplasmático (p: 0.002). Anti-Ro52+ aislado se asoció negativamente con el patrón moteado fino (p<0.001) y positivamente con el citoplasmático (p<0.001). La principal asociación con síntomas clínicos fue de xerostomía y xeroftalmia con anti-Ro52+/anti-Ro60+/anti-La+ (p<0.001), úlceras orales con anti-Ro52+/anti-Ro60+/anti-La− (p: 0.002) y alopecia con anti-Ro52−/anti-Ro60+/anti-La−. Asociaciones negativas fueron xeroftalmia y fotosensibilidad con anti-Ro52+/anti-Ro60−/anti-La− (p: 0.003). Asociaciones positivas de laboratorio fueron hipergammaglobulinemia con anti-Ro52+/anti-Ro60+/anti-La+ (p: 0.003) e hipocomplementemia con anti-Ro52−/anti-Ro60+/anti-La− (p: 0.003). Leucopenia se asoció negativamente con anti-Ro52+/anti-Ro60−/anti-La− (p: 0.003).
ConclusiónNuestro estudio encontró una relación significativa entre las manifestaciones clínicas y de laboratorio con diferentes patrones de anticuerpos anti-Ro52, anti-Ro60 y anti-La. La combinación de anticuerpos podría ser clínicamente útil, debido a implicaciones pronósticas y terapéuticas.
Autoimmune diseases represent a wide variety of clinical problems affecting multiple organs and systems, and affect as least 5% of the population.1 A great diversity of antibodies has been associated with different clinical manifestations and clinicians have relied on them guiding clinical diagnosis, prognostic implications, and in some cases therapeutic decisions.2,3 Current knowledge on pathogenesis of autoimmune diseases agrees that a complex interaction of genes and environmental features are needed for them to appear.4
Combination of line immunoblot ENA assay (INNOLIA-ANA) and indirect immunofluorescence techniques to detect antinuclear antibodies in HEp-2 cells as substrate are good screening methods in patients with a clinical suspicion of an autoimmune disease, mainly systemic lupus erythematosus (SLE) and Sjögren's syndrome (SS). Although false positive results can occur, titers >1:80 suggest the possibility of an autoimmune disorder and should prompt ordering more specific evaluations to determine specific reactivities of the antinuclear antibodies (ANA) such as anti-double stranded DNA and extractable nuclear antigens (ENA).5
Ro/SSA and La/SSB are heterogeneous antigenic complexes formed by three different proteins (Ro-52, Ro-60 and La) and four YRNA particles. The Ro60 protein acts as a quality check point for RNA misfolded with molecular chaperones for defective RNAs. The misfolded RNAs are recognized and then tagged by Ro60 for degradation. Ro52 interacts with different molecules, among them calreticulin and the immunoglobulin heavy chain-binding protein. Ro52 is thought to modify the role or stability of its substrates through ubiquitination, and this modification might result in the Ro52 mediated biological events.6
Anti-Ro/SSA and anti-La/SSB antibodies have been described in various autoimmune diseases. In primary Sjögren's syndrome (pSS) circulating antibodies are detected in approximately 60–70% of patients, and higher levels have been associated with early disease onset and systemic manifestations.7,8 GEMESS Study Group, which included 12 reference centers in Spain, confirmed these clinical and laboratory features in a cohort of patients with pSS, and observed decreased levels of C4 in patients with early disease onset.9
Anti-Ro/SSA are detected in 30% of patients with SLE diagnosis, particularly (90%) those subtypes with late onset, subacute cutaneous lupus erythematosus, drug induced lupus and congenital deficiencies of C2, C4 and C1q, also in patients with SS/SLE overlap syndrome and undifferentiated connective tissue disease.10 In contrast, anti-La/SSB is more commonly associated with SS,11 and strongly correlated in anti-La/SSB positive with anti-Ro/SSA negative to organ dysfunction (kidney, lung, liver).12
Since the eighties, development of congenital heart blockade has been described in autoimmune diseases, such as SS and SLE. However cardiac involvement is more related to circulating antibodies from the mother rather than the diagnosis of autoimmune disease. Although the immune profile is variable among different studies the anti-Ro52 antibody is more common in mothers of children with congenital cardiac blockade, neonatal lupus, and neonates with prolonged QT without congenital cardiac blockade.10,13,14
Prevalence and clinical associations of anti-Ro/SSA and anti-La/SSB antibodies may vary in different ethnic groups. In addition to studies conducted in the Spanish population on immune expression of SS, other studies have been developed in recent years on the immune profile of patients with anti-Ro52, anti-Ro60 and anti-La antibodies. Most of them have been performed on specific diagnosis of connective tissue disorders. In a cohort of patients with pSS developed in Korea associations of these antibodies with different clinical manifestations were observed. Anti-Ro52 was the most frequent autoantibody and related with liver and muscle damage.15 A recent study in the Mexican population to determine the prevalence of pSS in recent-onset SLE showed the presence of anti-Ro/SSA as a predictor of overlap, while the absence of anti-Ro/SSA, anti-La/SSB and rheumatoid factor was related to the lowest risk of overlap.16 Finally, the Ghent University Hospital proposed a study to determine the diagnostic distribution associated with serotype of anti-Ro/SSA or anti-La/SSB in consecutive patients referred to the rheumatology laboratory. SLE was associated to anti-Ro60 positive and isolated anti-La predisposed more to pSS.17
We undertook this study to evaluate clinical, laboratory and diagnosis associations of this important subset of antibodies at a hospital in Spain.
MethodsResearch centerHospital de Jerez de la Frontera is a regional specialty center located in Southwestern Spain. It provides specialized clinical services and has 550 beds. Its Immunology Department analyzes around 5800 ANA test and 1700 immunoblot ENA assay per year in a population of 450,000 inhabitants. Among its laboratory personnel are a biologist and an immunologist who perform specialized tests for different departments. Any practicing clinician can order antinuclear antibodies tests but they are mainly requested by rheumatologists, followed by primary care physicians, gastroenterologists, internists, nephrologists, pneumologists and hematologists.
SamplesA computerized registry of laboratory results is available since June 2002. We retrospectively analyzed all consecutive samples ordering ANA test from June 2002 to December 2012. Sera with titter at least 1:160 by immunofluorescence assay on HEp-2 cells (Euroimmun, Germany) were considered positive and performed by a single trained observer. Those sera with a positive ANA test for this titer dilution were analyzed using a commercially available line immunoblot ENA assay (INNO-LIA ANA, Fujirebio, Japan). This kit provides a qualitative in vitro assay for human autoantibodies of the IgG class to 13 different antigens: RNP-70, RNP-A, RNP-C, Ro-52, Ro-60, La/SSB, Scl-70, CENP-B, histones, Jo-1, Sm, P ribosomal protein and U1-nRNP complex in serum or plasma. The overall sensitivity and specificity of autoantibody detection by LIA was similar or higher as compared to combined conventional techniques.18,19 This immunoblot has 99.6% specificity to anti-La, whereas anti-Ro60 has 98.2%, and anti-Ro52 has 98.3%.20
Furthermore the computer system allow us to detect in the immunoassay anti-Ro and/or anti-La positive with negative ANA test, because in patients with high clinical suspicion of an autoimmune disease the immunoblot ENA assay was performed despite a negative ANA test. Finally, only patients with more than one positive serum samples for anti-Ro/SSA or anti-La/SSB were included in the study.
Clinical and laboratory data were obtained by either two trained observers by reviewing clinical charts or a computerized laboratory data base.
VariablesDependent variables were single or combined positives for anti-Ro52, anti-Ro60 and anti-La antibodies and all possible combinations were characterized as subgroups for further analysis, e.g. anti-Ro52+/anti-Ro60+/anti-La+, anti-Ro52+/anti-Ro60+/anti-La−, anti-Ro52+anti-Ro60−/anti-La+, anti-Ro52+/anti-Ro60−/anti-La−, anti-Ro52−/anti-Ro60+/anti-La+, anti-Ro52−/anti-Ro60+/anti-La−, anti-Ro52−/anti-Ro60−/anti-La+.
Independent variables included demographics (age, gender), clinical (diagnosis, disease characteristics), laboratory (hemoglobin, leukocyte and platelet counts, thyroid hormone levels) or immunologic (complement levels, hypergammaglobulinemia, rheumatoid factor).
Statistical analysisDescriptive statistics were used with frequencies and percentages for qualitative variables and mean, standard deviation, and range for quantitative variables. Clinical associations between different sets of positive combinations against clinical or laboratory variables were analyzed by contingency tables using chi square of Fisher's exact tests. In all cases alpha level was set at 0.01 as an adjustment for multiple comparisons. Strength of association is presented as odds ratio and its 99% confidence intervals. We performed the cluster analysis of variables using average linkage between groups, and using as interval measure, the squared Euclidean distance. Analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL).
ResultsA total of 41,102 serum samples were referred to the Department of Immunology for ANA detection. 12,124 samples were positive from 2970 patients. 322 patients with more than one positive serum sample for anti-Ro52 and/or anti-Ro60 and/or anti-La antibodies with titer dilution >160 were included. Females represented 90.4% of the sample. They had an age between 10 and 84, mean 44.4 and standard deviation 15.7 years. Some isolated sera were positive for immunoblot ENA assay with negative ANA test. But the 322 patients showed all or most of their sera positive for ANA test, and the pattern of sera for each patients were virtually identical. No patient was found in our study with more than one sera negative test for IIF.
Main clinical diagnosis in descending order of frequency were: 129 SS (40.06%), 88 of them had a primary disease, 118 SLE (36.6%), 27 undifferentiated connective tissue disease (8.4%), 26 systemic sclerosis (8.1%) (included 9 CREST syndrome), 16 rheumatoid arthritis (4.9%), 11 discoid lupus (3.4%), 7 mixed connective tissue disease (2.2%) and 5 inflammatory myopathy (1.6%). Secundary Sjögren syndrome was found in 41 patients, mainly associated to SLE (30 patients) and rheumatoid arthritis (5 cases).
In relation to immunofluorescence patterns in HEp-2 cells the fine speckled pattern was the most common in 225 cases (69.8%) followed by homogenous pattern in 64 (19.8%), cytoplasmic pattern in 36 (11.1%), coarse speckled pattern in 23 (7.1%), nucleolar in 18 (5.5%) and centromeric in 9 patients (2.8%). We found a mixed pattern in 53 patients, the most prevalent was homogeneus/fine speckled mixed pattern in 11 of them (3.4% of all patients).
Main clinical and laboratory data of patients with positive samples for anti-Ro52 and/or anti-Ro60 and/or anti-La (322 cases) are presented in Table 1. Specificities of different antigens of these patients are depicted in Table 2. We can see that the most frequent positive finding was anti-Ro52 in 269 patients (83.5%), followed by 205 patients with anti-Ro60 (63.7%) and 155 with anti-La (48.1%).
Main clinical and laboratory data of patients with positive samples for anti-Ro52 and/or anti-Ro60 and/or anti-La (322 cases).
| Frequency (%)/(n) | |
|---|---|
| Clinical features | |
| Xerostomia | 45.3 (146) |
| Xerophthalmia | 48.8 (157) |
| Arthritis | 41 (132) |
| Raynaud's phenomenon | 27.6 (89) |
| Photosensitivity | 27 (87) |
| Oral ulcers | 24.2 (78) |
| Alopecia | 18.3 (59) |
| Interstitial pneumonitis | 10.6 (34) |
| Acute cutaneous lupus | 10.6 (34) |
| Peripheral nervous system involvement | 9.3 (30) |
| Vasculitis | 7.5 (24) |
| Glomerulonephritis | 6.5 (21) |
| Central nervous system involvement | 6.5 (21) |
| Pleural effusion | 5.9 (19) |
| Pericardial effusion | 5.6 (18) |
| Discoid lupus | 5.3 (17) |
| Subacute cutaneous lupus | 4 (13) |
| Heart blockade | 0.6 (2) |
| Laboratory features | |
| Rheumatoid factor | 43.5 (140) |
| Leukopenia | 37.3 (120) |
| Lymphopenia | 32.3 (104) |
| Hypergammaglobulinemia | 26.1 (84) |
| Anemia | 25.2 (81) |
| Hypocomplementemia | 24.5 (79) |
| Hypothyroidism | 11.5 (37) |
| Thrombocytopenia | 10.9 (35) |
Specificities of different antigens of patients with positive samples for anti-Ro52 and/or anti-Ro60 and/or anti-La (322 cases).
| INNOLIA-updated | Frequency (%)/(n) |
|---|---|
| Ro52 | 83.5 (269) |
| Ro60 | 63.7 (205) |
| La | 48.1 (155) |
| RNPA | 9.0 (29) |
| RNPC | 7.1 (23) |
| SmB | 6.5 (21) |
| Centromere | 5.9 (19) |
| SmD | 5.0 (16) |
| RNP70 | 4.7 (15) |
| Jo-1 | 4.0 (13) |
| Ribosomal | 2.8 (9) |
| Scl-70 | 1.6 (5) |
Table 3 shows different associations between combinations of antibodies anti-Ro52/anti-Ro60/anti-La and diagnosis, clinical or laboratory results. Table 4 shows significant associations of positive test for the presence of circulating antibody anti-Ro52, anti-Ro60 and anti-La. Since a strict significance level selected, some interesting associations were not presented such as the group anti-Ro52+ with cytoplasmic pattern (OR 3.72, 99% CI: 0.9–16, p value 0.04) and the group anti-Ro60+ with subacute cutaneous lupus (SCLE) (OR 7.0, 99% CI: 0.9–55.3, p: 0.023).
Associations between combinations of anti-Ro52, anti-Ro60 and anti-La antibodies and diagnosis, clinical or laboratory results.
| Anti-Ro/SSA and/or anti-La/SSB immunoblot ENA assay Frequency %/(n) | Association | p | OR | 99% Confidence interval | |
|---|---|---|---|---|---|
| Anti-Ro52+/anti-Ro60+/anti-La+ 36.3% (117) | Fine speckled pattern | <0.001 | 3.77 | 2.04 | 6.96 |
| Xerostomia | <0.001 | 2.51 | 1.57 | 4.01 | |
| Primary Sjögren | 0.001 | 2.29 | 1.38 | 3.79 | |
| Xerophthalmia | <0.001 | 2.27 | 1.42 | 3.62 | |
| Rheumatoid factor + | 0.001 | 2.17 | 1.36 | 3.46 | |
| Hypergammaglobulinemia | 0.003 | 2.14 | 1.28 | 3.56 | |
| Homogeneous pattern | 0.002 | 0.38 | 0.20 | 0.75 | |
| Cytoplasmic pattern | 0.002 | 0.25 | 0.09 | 0.68 | |
| Systemic sclerosis | 0.004 | 0.21 | 0.06 | 0.73 | |
| Anti-Ro52+/anti-Ro60+/anti-La− 17.0% (55) | Oral ulcers | 0.002 | 2.59 | 1.39 | 4.80 |
| Arthritis | 0.006 | 2.22 | 1.23 | 4.03 | |
| SLE | 0.009 | 2.13 | 1.18 | 3.84 | |
| Anti-Ro52+/anti-Ro60−/anti-La+ 4.3% (14) | Rheumatoid factor + | 0.008 | 4.97 | 1.36 | 18.19 |
| Anti-Ro52+/anti-Ro60−/anti-La− 26.7% (86) | Systemic sclerosis | <0.001 | 11.84 | 4.56 | 30.72 |
| Nucleolar pattern | <0.001 | 11.66 | 3.71 | 36.59 | |
| CREST | 0.002 | 10.54 | 2.14 | 51.82 | |
| Cytoplasmic pattern | <0.001 | 5.62 | 2.72 | 11.63 | |
| Centromeric pattern | <0.001 | 4.13 | 3.39 | 5.04 | |
| Xerophthalmia | 0.003 | 0.47 | 0.28 | 0.78 | |
| Leukopenia | 0.003 | 0.43 | 0.23 | 0.78 | |
| Photosensitivity | 0.003 | 0.40 | 0.20 | 0.76 | |
| Rheumatoid factor + | <0.001 | 0.39 | 0.22 | 0.67 | |
| SLE | <0.001 | 0.30 | 0.16 | 0.56 | |
| Fine speckled pattern | <0.001 | 0.26 | 0.15 | 0.45 | |
| Anti-Ro52−/anti-Ro60+/anti-La− 7.4% (24) | Anti-RNP C | <0.001 | 7.73 | 2.78 | 21.48 |
| Anti-RNP 70 | 0.002 | 8.02 | 2.48 | 25.97 | |
| Alopecia | 0.003 | 3.90 | 1.62 | 9.41 | |
| Hypocomplementemia | 0.003 | 3.77 | 1.59 | 8.94 | |
| Oral ulcers | 0.009 | 3.16 | 1.33 | 7.48 | |
| Anti-Ro52−/anti-Ro60−/anti-La+ 4.0% (13) | No association found for p value<0.01 | ||||
SLE, systemic lupus erythematosus.
Associations between presence of circulating anti-Ro52+, anti-Ro60+ and anti-La+ antibodies and clinical and laboratory data.
| Anti-Ro/SSA and/or anti-La/SSB immunoblot ENA assay | Association | p | OR | 99% Confidence interval | |
|---|---|---|---|---|---|
| Anti-Ro52+ | Xerostomia | 0.001 | 3.03 | 1.55 | 5.92 |
| Xerophthalmia | 0.002 | 2.54 | 1.35 | 4.79 | |
| Anti-RNP C | <0.001 | 0.17 | 0.07 | 0.43 | |
| Anti-Ro60+ | Anti-La | <0.001 | 5.54 | 3.31 | 9.27 |
| SLE | <0.001 | 3.28 | 1.23 | 5.55 | |
| Fine speckled pattern | <0.001 | 2.57 | 1.56 | 4.25 | |
| Undifferentiated connective tissue | 0.002 | 5.03 | 1.48 | 17.11 | |
| Xerophthalmia | 0.004 | 1.92 | 1.21 | 3.06 | |
| Cytoplasmic pattern | <0.001 | 0.24 | 0.11 | 0.50 | |
| Systemic sclerosis | <0.001 | 0.15 | 0.05 | 0.37 | |
| Nucleolar pattern | <0.001 | 0.14 | 0.04 | 0.44 | |
| Anti-La+ | Anti-Ro60 | <0.001 | 5.54 | 3.31 | 9.27 |
| Fine speckled pattern | <0.001 | 3.55 | 2.08 | 3.06 | |
| Rheumatoid factor+ | <0.001 | 2.60 | 1.65 | 4.09 | |
| Hypergammaglobulinemia | <0.001 | 2.46 | 1.47 | 4.12 | |
| Primary Sjögren | <0.001 | 2.38 | 1.43 | 3.95 | |
| Xerostomia | <0.001 | 2.20 | 1.40 | 3.44 | |
| Xerophthalmia | <0.001 | 2.17 | 1.39 | 3.39 | |
| Cytoplasmic pattern | <0.001 | 0.22 | 0.09 | 0.53 | |
| Systemic sclerosis | <0.001 | 0.15 | 0.05 | 0.37 | |
| Anti-RNP 70 | 0.001 | 0.07 | 0.01 | 0.54 | |
| Oral ulcers | 0.005 | 0.48 | 0.28 | 0.82 | |
| Anti-RNP A | 0.005 | 0.31 | 0.12 | 0.74 | |
| Nucleolar pattern | 0.005 | 0.20 | 0.05 | 0.71 | |
| Anti-RNP C | 0.007 | 0.27 | 0.10 | 0.76 | |
| Homogeneous pattern | 0.009 | 0.48 | 0.27 | 0.86 | |
SLE, systemic lupus erythematosus.
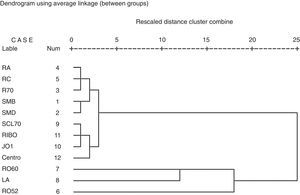
Using cluster analysis, four groups of autoantibodies can be identified. Cluster 1 comprises antibodies against SmB, SmD, RNP,70, RNP-A and RNP-C. Cluster 2 is formed for antibodies against Scl-70, Ribosomal P, Jo1 and centromere. Cluster 3 is formed for antibodies to Ro60 and SSB. Cluster 4 consisted of antibodies against Ro52 (Fig. 1).
DiscussionRheumatologists have established the importance of clinical and serological findings in classifying many autoimmune diseases. Some concerns have been published on the importance of critical evaluation of specialized tests in clinical care. It seems that ordering many tests could produce multiple laboratory results that must be carefully evaluated in the context of clinical findings.17,21
In our study we describe the clinical, diagnostic and immunological associations with all several possible combinations of anti-Ro52, anti-Ro60 and anti-La antibodies, found in a large cohort of patients from a single center in Spain for 10 years. A total of 322 patients presented more than one positivity for these antibodies, and the most prevalent disease were SLE and pSS, mainly associated with immunologic profile anti-Ro52+/anti-Ro60+/anti-La− and anti-Ro52+/anti-Ro60+/anti-La+, respectively.
Our strict definition of statistical significance could make that some associations were lost but we are confident that described associations could be more robust. The clinical and practical relevance of these associations could be particularly interesting for isolated anti-Ro52 (anti-Ro52+/anti-Ro60−/anti-La−). This subset of autoantibodies has independent clinical and immunological associations, data confirmed in our cluster analysis.22,23 Positive isolated anti-Ro52 antibody was associated with systemic sclerosis, especially those with CREST syndrome, and dermatomyositis (OR 11.65, 99% CI: 1.28–105.79, p: 0.018). Other associations were mainly seen with some laboratory findings such as anti-Jo-1 (OR 3.45, 99% CI: 1.12–10.59, p: 0.03), anti-centromere (OR 2.66, 99% CI: 1.04–2.80, p: 0.03) or particular immunofluorescence patterns such as centromeric, cytoplasmic and nucleolar pattern. Moreover, comparing the isolated positive anti-Ro52+ group (anti-Ro52+/anti-Ro60−/anti-La−) with positive triple reactivity (anti-Ro52+/anti-Ro60+/anti-La+) the clinical, laboratory and diagnosis associations found in one group were the opposite in the other one. So, the immunologic subset anti-Ro52+/anti-Ro60+/anti-La+ was positively associated to xerostomia and fine speckled pattern and negatively to systemic sclerosis (OR 0.22, 99% CI: 0.05–1.00, p: 0.026). This highlights the diagnostic clinical value of the combination. In relation to positive test result for the group of anti-Ro60+, mainly associated with anti-La− (anti-Ro52+/anti-Ro60+/anti-La− and anti-Ro52−/anti-Ro60+/anti-La−), was very indicative for SLE.
Our findings are similar to those previously reported.6,22–25 The presence of circulating antibody anti-Ro52, anti-Ro60 and anti-La positive predisposed to xerostomia and xerophthalmia (xerostomia in anti-Ro60 OR 1.75, 99% CI: 1.10–2.79, p: 0.012). This clinical data supported by numerous studies on the pSS.6–9,11,17,24 Positive anti-Ro52 with negative anti-Ro60 and anti-La exhibited negative association with photosensitivity, however in previous studies patients with anti-Ro52+ had higher frequency of cutaneous involvement.17,26 Furthermore, this immunological combination (anti-Ro52+/anti-Ro60−/anti-La−) was also inversely associated with xerostomia and xerophthalmia. However in previous studies isolated positive anti-Ro52 was closely related with the main clinical, histopathological and immunological features of pSS.27 Isolated positive anti-Ro60 or anti-Ro60+ combined with anti-Ro52+ increased the probability for SLE or overlap SLE/SS, as described in several works,11,17,28 but also in our cohort was strongly associated to oral ulcers and arthritis. Anti-La reactivity was strongly associated to pSS and its main clinical manifestations (xerostomia and xerophthalmia), as in many others studies in different populations.11,17,26
Most previous research studies on the usefulness of these antibodies have been performed on samples from patients with a diagnosed autoimmune disease, especially SLE and SS. However there are few studies performed to describe the diagnostic association of anti-Ro and anti-La antibodies identified in a consecutive samples.17
In addition to diagnosis, we determined the utility of the immunologic profiles with anti-Ro52 and/or anti-Ro60 and/or anti-La in the prediction of clinical and laboratory data in a consecutive sample of patients and these are ordered for all departments of hospital and primary care. We reviewed all the samples of each patient during this period and registered the more prevalent anti-Ro52, anti-Ro60 and anti-La reactivity combination. The selected positivity cut-off point >160 increases the validity of the study, due to the positive ANA test and anti-Ro/SSA and anti-La/SSB assay can be detected in up to 25–30% and 1.6% of healthy individuals, respectively. Their prevalence is higher with age, and its positivity without a relevant clinical data can be misleading.29
Therefore, in our study we showed an immune profile may lead us to a certain diagnosis or development of disease manifestations. This is more important in the onset of autoimmune diseases, due to circulating antibodies may be present several years before disease diagnosis, and could indicate the severity of its manifestations.17,30
Some limitations inherent to these studies are the retrospective clinical evaluations of clinical charts and the possible diagnostic biases in treating physicians who see these patients and order specialized laboratory tests. Many of our patients with positive ANA test resulted negative for the immunoblot ENA assay determination. This could be related to the type of immunoassay developed. In cases with a high clinical suspicion specific antibody detection is recommended.11
Ethical disclosuresProtection of human and animal subjectsThe authors declare that this research has not been conducted experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of the workplace on the publication of patient data.
Right to privacy and informed consentThe authors declare that this article does not appear patient data.
Conflict of interestThe authors declare no conflict of interest.