The effect of overweight/obesity on clinical status in rheumatoid arthritis (RA) is still a controversial topic.
AimTo assess the association between body composition and clinical status in RA patients.
MethodsA prospective, comparative, cross-sectional study was performed on 123 (98.4% women, 86.3% FR+, 9.3±8.7 duration years) RA patients diagnosed according to ACR/EULAR 2010 criteria who were assessed for inflammatory activity (DAS 28), functional status (HAQ-Di), and type of treatment. Body composition was evaluated by BMI, waist, hip, and middle arm girths, waist/hip ratio, skin fold measurements, and bioelectrical impedance analysis.
ResultsThe prevalence of overweight and obesity (BMI-WHO cut-off points) was 30.9% and 45.5% respectively. Using Stavropoulos-Kalinoglou cut-off points, each corresponding prevalence increased to 31.7% and 58.5%, respectively. Pooled patients in the overweight/obesity classification (Stavropoulos-Kalinoglou classification) exhibited a significantly higher number of swollen joints as compared to subnormal/normal body composition subjects (3.8±3.3 vs 1.9±2.5; P=.02). Swollen joint count showed significant positive correlation with 6 out of 11 body composition parameters: BMI; arm and hip girths, triceps skin fold, body fat average determined by bioelectrical impedance analysis, and skin fold measurements.
ConclusionsPrevalence of obesity in RA varies according to BMI cut-off points. Overweight and obesity were associated with higher inflammatory activity characterised by a higher count of tender and swollen joints. A positive correlation was found between swollen joint amount and the majority of the body fat mass indicators assessed. Body composition assessment/improvement should be an important part of the routine care of RA patients.
La asociación entre la presencia de sobrepeso/obesidad y el estado clínico de la artritis reumatoide (AR) es un tema aún no resuelto.
ObjetivoEvaluar la asociación entre el tipo de composición corporal y el estado clínico en pacientes con AR.
MétodosEstudio prospectivo, comparativo y transversal que incluyó a 123 pacientes (98,4% mujeres, 86,3% FR+, 9,3±8,7 años de duración) con AR (criterios ACR/EULAR 2010) en quienes se determinó actividad inflamatoria (DAS 28), estado funcional (HAQ-Di) y tipo de tratamiento; además, el tipo de composición corporal evaluada por IMC, circunferencias de cintura, cadera y brazo medio, índice cintura/cadera, plicometría y bioimpedancia eléctrica.
ResultadosLas prevalencias de sobrepeso y obesidad (IMC-OMS) fueron del 30,9% y del 45,5%. Cuando se reclasificaron mediante los puntos de corte de Stavropoulos-Kalinoglou, las prevalencias aumentaron a 31,7 y 58,5%, respectivamente. Con este criterio, los pacientes con sobrepeso/obesidad tuvieron más articulaciones inflamadas que los pacientes con composición corporal subnormal/normal (3,8±3,3 vs 1,9±2,5; p=0,02). El conteo de articulaciones inflamadas mostró correlación positiva significativa con 6 de 11 métodos antropométricos: IMC, circunferencia de brazo y cadera, pliegue tricipital y porcentaje de grasa corporal (determinado por bioimpedancia eléctrica y plicometría).
ConclusionesEl sobrepeso y la obesidad se asociaron a mayor actividad inflamatoria caracterizada por mayor cantidad de articulaciones inflamadas. Encontramos correlación positiva significativa entre el número de articulaciones inflamadas y la mayoría de los indicadores de masa grasa corporal estudiados. La evaluación y optimización de la composición corporal podría llegar a ser una parte importante para el abordaje clínico de pacientes con AR.
Rheumatoid arthritis (RA) is an autoimmune disease that is chronic and generalised, based on the inflammation and hyperplasia of the synovial membrane. It naturally progresses towards structural disruption, which in turn leads to musculoskeletal deformity, disability and a reduction in life expectancy, all of which are correlated with the level of inflammation.1
In the language of clinical nutrition, the expression “body composition” is associated with the evaluation of different components within the body, and it may refer to different levels such as the anatomical, molecular, cellular and tissue levels, or the total body.2,3 The determination of body composition at tissue level by using anthropometric techniques, such as the body mass index (BMI), skin fold measurements, waist or hip circumference and bio-electrical impedance analysis (BEI), makes it possible to gauge nutritional status as well as the percentage of body fat, energy reserve and the risk of diseases associated with alterations of these parameters.4,5
An increasing amount of scientific evidence shows that alterations in bodily composition characterised by an increase in the mass of fat, as well as heightening the probability of cardiovascular, metabolic and neoplastic diseases, may also play a role in modulating certain inflammatory diseases.6 In states of hyperadiposity, the adipocytes display hypertrophy and a status of activation characterised by the liberation of increased amounts of proinflammatory soluble mediators (adipocynes), such as leptin and resistin, giving rise to the proliferation and differentiation of mononuclear phagocytes system cells and the activation of the natural killer cells. Moreover, activated adipocytes in states of hyperadiposity have been shown to be a source of other proinflammatory cytokines such as chemerin, the retinol-4 transporter protein, lipocalin and, importantly for RA, TNFα, IL-6 and IL-12.7–9 Given that IL-1 as well as TNF are fundamental for the creation of chronic inflammation and locally destructive synovial proliferation, which are the aetiopathogenic basis of RA, study of the modulation of the inflammatory activity of RA by the level of body fat is a potentially interesting aspect for the study and clinical treatment of patients with RA. Although this subject has been covered by several previous studies, their results in this respect are contradictory.10,11 This may be explained by design weakness, the different evaluation methods used—for body composition as well as the level of the inflammatory activity of RA—or low statistical power. These methodological aspects may be detected in the majority of the reports on study of this subject.12
The above arguments clearly show that there is a relationship between alterations in bodily composition characterised by an increase in the amount of fat, such as being overweight or obese, and the level of inflammatory activity in patients with RA. This relationship has yet to be explained scientifically, so the aim of this study was to evaluate the degree of association between the physical composition and clinical status of RA patients. We used several anthropometric methodologies for this purpose, characterising body type and evaluating the clinical status of RA by measuring the degree of inflammatory activity, the level of physical functioning and the type of treatment required.
Material and methodsPatientsThis study was transversal, and the target population was made up of occasional or regular patients with RA treated in the Rheumatology Department of the Regional General Hospital, the Social Services and Security Institute for State Workers, which is a tertiary care unit in Merida (Yucatan, Mexico). The inclusion criteria were: RA according to the ACR/EULRA 2010 classification criteria13 and age older than 18 years old. The sample was obtained when convenient and was composed of mixed-race Mexicans. No subjects with RA who had any other disorder that could cause an altered bodily composition were included, such as active neoplastic disease, chronic infection, malabsorption or endocrine disorders. Subjects who withdrew their consent were excluded, as were those whom it was impossible to measure clinically and anthropometrically.
Measurement of the clinical status of rheumatoid arthritisThe clinical status of RA was evaluated using 3 different aspects: level of inflammatory activity, which was evaluated using the 4 element DAS 28-ESR method14; we also determined the level of physical functioning using a version of the HAQ-Di instrument adapted for use in the Mexican population,15 and lastly we recorded the main aspects of pharmacological treatment, such as the use and dose of prednisone (PDN) and the use of synthetic and biological antirheumatic drugs which modify the disease. Clinical variables were obtained in a clinical interview and by review of the patient's clinical file on the same day. These were recorded by 3 independent researchers (RLV, DQG and LMV) who received training on how to standardise information prior to starting the study.
Anthropometric measurementsBody composition was evaluated by focussing on 7 different anthropometric measurements: BMI, waist, hips and average arm circumference; the waist/hip ratio, skin fold thickness (triceps, biceps, subscapular and iliac) and BEI. The anthropometric measurements were taken according to the Official Mexican Norm by a single researcher (MGS), who is a qualified expert for the exercise of Clinical Nutrition and who was blinded for the clinical study. All anthropometric measurements were obtained within a period of time no longer than 48h after the clinical interview.
Body composition was classified (as below normal, normal, overweight and obese) based on BMI figures in 2 different ways: according to the cut-off points recommended by the World Health Organisation (BMI-WHO) and by using the criteria published by Stavropoulos-Kalinoglou et al., as these authors16 have offered evidence that the BMI cut-off points recommended by the WHO may not be suitable for categorising body composition in subjects with RA. We therefore also classified body composition type according to this system, considering a BMI score higher than 23 to be overweight, and BMI scores higher than 28 to correspond to obesity.
Statistical analysisNumerical variables were compared using the unpaired t-test or one-way ANOVA, depending on the number of groups. The primary analytical variable was the DAS 28 score. Additionally, the correlation between the numerical variables (anthropometric, HAQ-Di, inflammatory activity and PDN dose) was evaluated using Pearson's coefficient. χ2 with Yates’ correction or Fisher's exact test was used to compare categorical variables. Statistical analysis was undertaken using version 20.0 of the SPSS statistical package (IBM Corp.). The threshold for statistical significance was set at 0.05.
Ethical considerationsThe protocol was approved by the Research Committee of Universidad Anáhuac Mayab and the Ethics and Research Committees of the Hospital Regional de Alta Especialidad de la Península de Yucatán (registration number 2014-31). The Helsinki Declaration was taken into consideration. All of the subjects included signed the informed consent form before being included in the study. All of the patients included received basic nutritional guidance and all of the overweight or obese patients were encouraged to seek nutritional help from a qualified Clinical Nutrition professional.
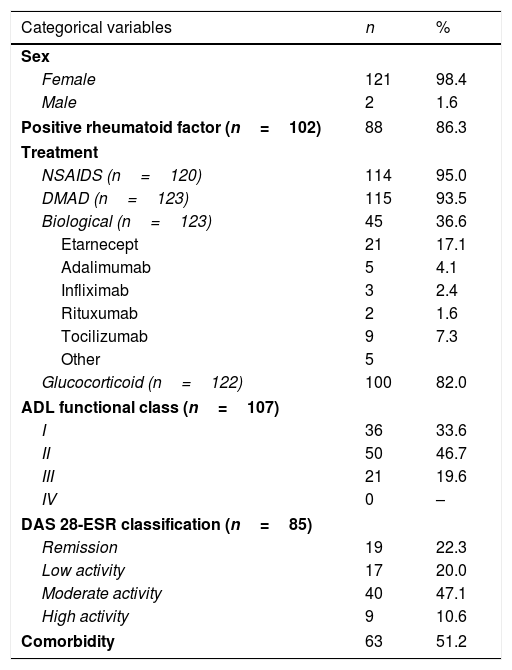
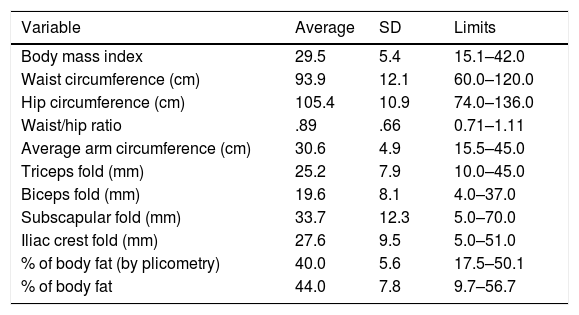
ResultsA total of 123 participants were selected (121 women and 2 men). The clinical and demographic characteristics of the participants are described in Table 1, and they all correspond to patients with RA treated in a tertiary hospital. The ESR-DAS 28 score could only be evaluated in 85 patients, as due to logistical reasons in 38 patients it was impossible to obtain the ESR values. As it was possible to measure the other individual parts of the DAS 28 in these 38 subjects (self-evaluation of their clinical state and number of painful and inflamed joints), the sample size used in the comparisons involving these individual components of the DAS 28 score amounted to 123. The results describing all of the anthropometric measurements are shown in Table 2.
Clinical and demographic profile of the patients studied.
| Categorical variables | n | % |
|---|---|---|
| Sex | ||
| Female | 121 | 98.4 |
| Male | 2 | 1.6 |
| Positive rheumatoid factor (n=102) | 88 | 86.3 |
| Treatment | ||
| NSAIDS (n=120) | 114 | 95.0 |
| DMAD (n=123) | 115 | 93.5 |
| Biological (n=123) | 45 | 36.6 |
| Etarnecept | 21 | 17.1 |
| Adalimumab | 5 | 4.1 |
| Infliximab | 3 | 2.4 |
| Rituxumab | 2 | 1.6 |
| Tocilizumab | 9 | 7.3 |
| Other | 5 | |
| Glucocorticoid (n=122) | 100 | 82.0 |
| ADL functional class (n=107) | ||
| I | 36 | 33.6 |
| II | 50 | 46.7 |
| III | 21 | 19.6 |
| IV | 0 | – |
| DAS 28-ESR classification (n=85) | ||
| Remission | 19 | 22.3 |
| Low activity | 17 | 20.0 |
| Moderate activity | 40 | 47.1 |
| High activity | 9 | 10.6 |
| Comorbidity | 63 | 51.2 |
| Numerical variables | Average | SD | Limits |
|---|---|---|---|
| Age (years) (n=123) | 46.8 | 13.1 | 19–79 |
| Duration of RA (years) (n=123) | 9.3 | 8.7 | .1–50.0 |
| Daily dose of PDN (mg) (n=100) | 5.2 | 1.3 | 2.5–12.5 |
| No. of painful joints (n=123) | 4.9 | 4.9 | 0–22 |
| No. of inflamed joints (n=123) | 3.7 | 3.3 | 0–16 |
| ESR (mm/h) (n=85) | 28.5 | 20.1 | 5.0–94.0 |
| DAS 28 (n=85) | 3.6 | 1.1 | .7–6.5 |
| HAQ-Di classification (n=123) | .52 | .43 | .00–1.80 |
NSAIDS: non-steroid anti-inflammatory drugs; DMAD: disease modifying antirheumatic drugs.
Results of evaluation of body composition in the study group (n=123).
| Variable | Average | SD | Limits |
|---|---|---|---|
| Body mass index | 29.5 | 5.4 | 15.1–42.0 |
| Waist circumference (cm) | 93.9 | 12.1 | 60.0–120.0 |
| Hip circumference (cm) | 105.4 | 10.9 | 74.0–136.0 |
| Waist/hip ratio | .89 | .66 | 0.71–1.11 |
| Average arm circumference (cm) | 30.6 | 4.9 | 15.5–45.0 |
| Triceps fold (mm) | 25.2 | 7.9 | 10.0–45.0 |
| Biceps fold (mm) | 19.6 | 8.1 | 4.0–37.0 |
| Subscapular fold (mm) | 33.7 | 12.3 | 5.0–70.0 |
| Iliac crest fold (mm) | 27.6 | 9.5 | 5.0–51.0 |
| % of body fat (by plicometry) | 40.0 | 5.6 | 17.5–50.1 |
| % of body fat | 44.0 | 7.8 | 9.7–56.7 |
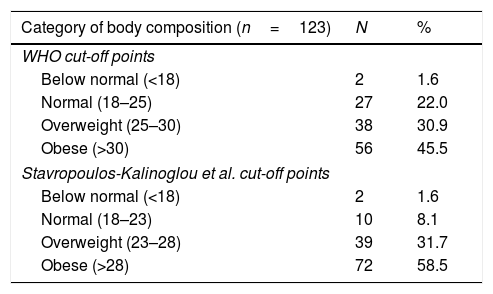
Body composition type classification according to BMI scores are shown in Table 3. According to the cut-off points of the BMI-WHO, 76.4% of the patients were classified as having hyperadiposity: 30.9% of them were classified as overweight and 45.5% as obese. When the BMI classification of body composition was re-evaluated using Stavropoulos-Kalinoglou cut-off points, the prevalence of patients classified as having a normal BMI fell from 22.0% to 8.1%, giving rise to a redistribution of subjects categorised as overweight. Nevertheless, the net prevalence of overweight subjects did not change, because a high proportion of subject previously classified as overweight according to WHO were reclassified as obese using the Stavropoulos-Kalinoglou cut-off points. The net result of this reclassification was a marked increase in the prevalence of obesity (58.5%).
Classification of body composition type according to BMI results.
| Category of body composition (n=123) | N | % |
|---|---|---|
| WHO cut-off points | ||
| Below normal (<18) | 2 | 1.6 |
| Normal (18–25) | 27 | 22.0 |
| Overweight (25–30) | 38 | 30.9 |
| Obese (>30) | 56 | 45.5 |
| Stavropoulos-Kalinoglou et al. cut-off points | ||
| Below normal (<18) | 2 | 1.6 |
| Normal (18–23) | 10 | 8.1 |
| Overweight (23–28) | 39 | 31.7 |
| Obese (>28) | 72 | 58.5 |
A tendency towards higher scores for the individual components of the DAS 28 score—such as self-evaluation by the patients and their number of inflamed and painful joints—as well as higher HAQ-Di scores and a higher proportion of patients categorised as obese who required biological therapies—based on WHO criteria or those proposed by Stavropoulos-Kalinoglou et al.—in comparison with subjects who are overweight or categorised as normal or below normal weight. However, none of these comparisons attained statistical significance (data not shown).
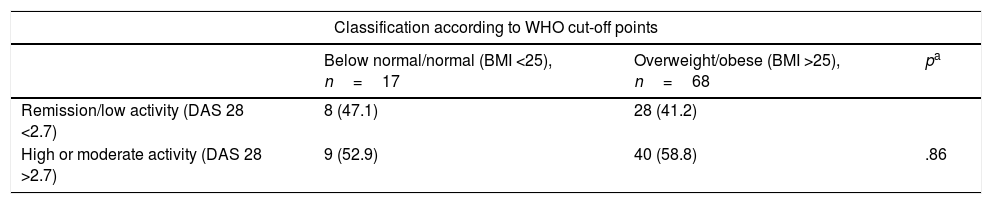
Based on the hypothesis that this lack of statistical significance could be due to a type ii error arising from the small size of the subsamples in subjects classified as normal or below normal weight, a post hoc analysis was performed which distributed the categories of body composition in a dichotomy: one category was composed of subjects classified as below normal and normal weight, while the other grouped the subjects classified as overweight and obese. Using this approach, we firstly found no statistically significant association between the categories of level of inflammatory activity evaluated by DAS 28 score and the type of body composition. This was the case for the WHO recommended BMI cut-off points as well as for those used by Stavropoulos-Kalinoglou et al. (Table 4).
Evaluation of the association between dichotomised body composition categories (according to the BMI) and inflammation status evaluated by the DAS 28-ESR*.
| Classification according to WHO cut-off points | |||
|---|---|---|---|
| Below normal/normal (BMI <25), n=17 | Overweight/obese (BMI >25), n=68 | pa | |
| Remission/low activity (DAS 28 <2.7) | 8 (47.1) | 28 (41.2) | |
| High or moderate activity (DAS 28 >2.7) | 9 (52.9) | 40 (58.8) | .86 |
| Classification according to Stavropoulos-Kalinoglou et al. cut-off points | |||
|---|---|---|---|
| Below normal/normal (BMI <23), n=7 | Overweight/obese (BMI >23), n=78 | pa | |
| Remission/low activity (DAS 28 <2.7) | 5 (71.4) | 31 (39.7) | |
| High or moderate activity (DAS 28 >2.7) | 2 (28.6) | 47 (60.3) | 0.22 |
Percentages are shown in brackets.
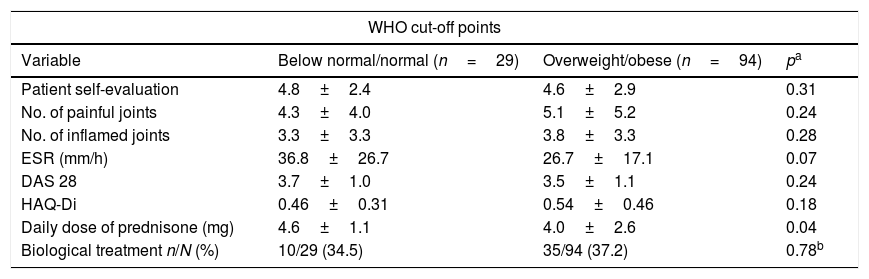
On the other hand, Table 5 shows a dichotomised comparison between type of body composition according to BMI values (below normal/normal weight and overweight/obesity) and the values of the clinical variables studied. This time the subcomponents of the overall DAS 28 scores were compared as well as the overall scores. Although no difference was found when using the cut-off points recommended by the WHO, a notable finding is that when the cut-off points recommended by Stravropoulos-Kalinouglu et al. were used, the overweight/obese patients were found to show statistically significant higher scores for inflamed joints as well as a clear tendency towards a higher number of painful joints (at the limit for statistical significance), in comparison with patients classified as being of below normal or normal weight.
Comparison between degrees of inflammatory activity, physical function and type of treatment, according to dichotomised BMI body composition categories.
| WHO cut-off points | |||
|---|---|---|---|
| Variable | Below normal/normal (n=29) | Overweight/obese (n=94) | pa |
| Patient self-evaluation | 4.8±2.4 | 4.6±2.9 | 0.31 |
| No. of painful joints | 4.3±4.0 | 5.1±5.2 | 0.24 |
| No. of inflamed joints | 3.3±3.3 | 3.8±3.3 | 0.28 |
| ESR (mm/h) | 36.8±26.7 | 26.7±17.1 | 0.07 |
| DAS 28 | 3.7±1.0 | 3.5±1.1 | 0.24 |
| HAQ-Di | 0.46±0.31 | 0.54±0.46 | 0.18 |
| Daily dose of prednisone (mg) | 4.6±1.1 | 4.0±2.6 | 0.04 |
| Biological treatment n/N (%) | 10/29 (34.5) | 35/94 (37.2) | 0.78b |
| Stavropoulos-Kalinoglou et al. cut-off points | |||
|---|---|---|---|
| Variable | Below normal/normal (n=12) | Overweight/obese (n=111) | pa |
| Patient self-evaluation | 3.4±2.2 | 4.7±2.8 | 0.06 |
| No. of painful joints | 2.7±3.0 | 5.1±5.0 | 0.05 |
| No. of inflamed joints | 1.9±2.5 | 3.8±3.3 | 0.02 |
| ESR (mm/h) | 35.3±20.9 | 27.4±20.1 | 0.19 |
| DAS 28 | 3.9±0.7 | 3.6±1.1 | 0.32 |
| HAQ-Di | 0.43±0.34 | 0.53±0.44 | 0.22 |
| Daily dose of prednisone (mg) | 4.3±1.5 | 4.2±2.4 | 0.40 |
| Biological treatment n/N (%) | 3/12 (25.0) | 42/111 (37.8) | 0.38b |
Results area shown as averages±SD.
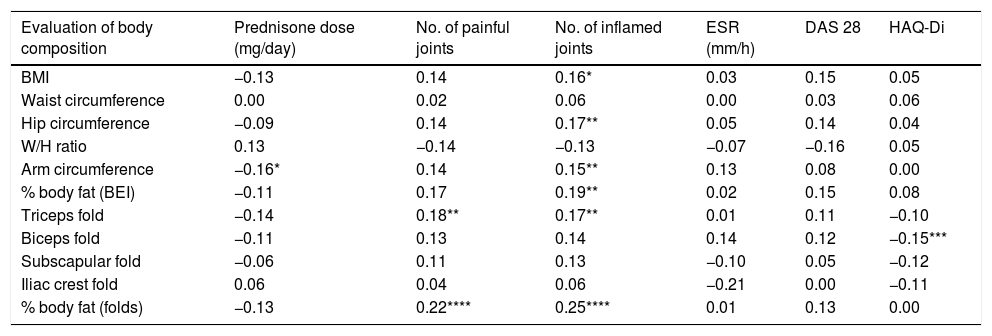
Table 6 shows the correlations between the different anthropometric measurements, the degree of inflammatory activity, the level of physical function and the required treatment. Notably, although the overall DAS 28 score shows no significant correlation with any of the methods studied for evaluating body composition, a significant positive correlation was found between the number of inflamed joints and 6 of the 11 anthropometric methods studied for body composition: BMI, hip and arm circumference, body fat measured by BEI and skin folds and triceps skin fold. The number of painful joints only showed a positive correlation with triceps skin fold and the percentage of body fat measured by plicometry. The daily dose of PDN required was negatively correlated with each anthropometric method, although it only attained statistical significance with average arm circumference. Finally, the HAQ-Di score correlated negatively with plicometric evaluations, although this was only statistically significant for the triceps fold.
Correlations (by Pearson coefficients) between the methods used to evaluate body composition and the level of inflammatory activity, physical function and the required treatment of RA.
| Evaluation of body composition | Prednisone dose (mg/day) | No. of painful joints | No. of inflamed joints | ESR (mm/h) | DAS 28 | HAQ-Di |
|---|---|---|---|---|---|---|
| BMI | −0.13 | 0.14 | 0.16* | 0.03 | 0.15 | 0.05 |
| Waist circumference | 0.00 | 0.02 | 0.06 | 0.00 | 0.03 | 0.06 |
| Hip circumference | −0.09 | 0.14 | 0.17** | 0.05 | 0.14 | 0.04 |
| W/H ratio | 0.13 | −0.14 | −0.13 | −0.07 | −0.16 | 0.05 |
| Arm circumference | −0.16* | 0.14 | 0.15** | 0.13 | 0.08 | 0.00 |
| % body fat (BEI) | −0.11 | 0.17 | 0.19** | 0.02 | 0.15 | 0.08 |
| Triceps fold | −0.14 | 0.18** | 0.17** | 0.01 | 0.11 | −0.10 |
| Biceps fold | −0.11 | 0.13 | 0.14 | 0.14 | 0.12 | −0.15*** |
| Subscapular fold | −0.06 | 0.11 | 0.13 | −0.10 | 0.05 | −0.12 |
| Iliac crest fold | 0.06 | 0.04 | 0.06 | −0.21 | 0.00 | −0.11 |
| % body fat (folds) | −0.13 | 0.22**** | 0.25**** | 0.01 | 0.13 | 0.00 |
BEI: bioelectrical impedance; W/H: waist/hip; BMI: body mass index.
The results of this work show that using the BMI cut-off points proposed by Stavropoulos-Kalinoglou et al., RA patients classified as overweight or obese have a significantly higher number of inflamed joints. They also show a clear tendency towards having more painful joints than is the case for patients classified as being of normal or below normal weight. On the other hand, a significant positive correlation was found between the number of inflamed joints and the majority of the anthropometric methods used to evaluate body fat volume. Both results support the hypothesis that, in patients with RA, being overweight or obese is associated with an increase in inflammatory joint activity.
The effect of body composition type on patients’ clinical state and more specifically the degree of inflammatory activity in RA has been examined in several previous studies,10–12,17–19 although their conclusions are contradictory. Some reports found a significant association between increased inflammatory activity and the presence of adiposity. Nevertheless, other reports found no proven association between the percentage of fat in body mass and the level of inflammatory activity in RA.
Some factors may explain the above-mentioned discrepancies. Firstly, the sample size and statistical power of the reports in question seem to be the most common cause of the inconsistencies. In some reports based on large samples a significant correlation was found, while those with smaller samples found no association between the degree of inflammatory activity in RA and type of body composition. Moreover, although body composition was evaluated using the BMI in all of the papers that were reviewed, those which classified body composition used the WHO cut-off points. Rheumatoid cachexia is highly frequent in patients with RA, and it is characterised by inflammation or physical inactivity with loss of muscle mass and replacement of this by fatty tissue, with relative conservation of total body weight. Based on this concept Stavropoulos-Kalinoglou et al. offered evidence that using BMI cut-off points for the general population to classify body composition in subjects with RA may be an erroneous indicator of body fat volume in these patients, as it may under-estimate it. These authors suggest that to use BMI as an indicator of body composition in patients with RA, BMI scores over 23 should be classified as overweight, while obesity corresponds to scores over 28. The use of this classification for body composition leads to a net increase in the number of patients with RA within the limits of obesity.
The majority of studies that have covered the relationship between degree of inflammation in RA and body composition use the DAS 2820 index to measure the inflammatory status of RA. DAS 28 is a viable, trustworthy and valid instrument for evaluating the level of inflammation in patients with RA. It is universally accepted as a valuable clinical and research tool for this purpose. However, the results of our study give rise to certain concerns regarding the validity of the DAS 28 index when evaluating the inflammatory status of subjects with RA in the presence of excessive fatty tissue. This observation is supported by the finding that, regardless of the classification criteria used (WHO cut-off points or the ones used by Stavropoulos-Kalinoglou), in our patients the overall DAS 28 score and ESR values were not associated at all with type of body composition. These results differ widely from the statistical association we found between other individual components of the DAS 28, such as the number of inflamed joints and being overweight or obese according to the Stavropoulos-Kalinoglou et al. cut-off points. Due to all of the above points, it is encouraging to state that the acute phase response in patients with RA is reduced in some way when the percentage of body fat exceeds certain limits. This opens up a line of research open on the effects of fatty tissue and the acute response in patients with RA. If this is proven, calculating the DAS 28 should be adjusted depending on body composition type.
We consider that our study has certain methodological advantages in comparison with previous reports on research into the relationship between level of inflammatory activity and type of body composition in RA: we used 2 classification methods for body composition (WHO and Stavropoulos-Kalinoglou); we also include the individual components of DAS 28 as well as its overall score in the statistical analysis. We also examine the correlation between levels of inflammatory activity and body composition scores by using 6 other anthropometric methods apart from the BMI.
On the other hand, we admit that the transversal nature of our study design is a major limitation that hinders proving any causal relationship between the associations found. The difficulty of recording the DAS 28 score in all of the patients included also reduced the statistical power of our study. Together with these considerations, the fact that our study was composed of a majority of female subjects is an analytical distortion. The fact is that the majority of patients who visit the Rheumatology Department and who agreed to take part in the study are women. Lastly, we admit that the statistical significances reported here on the association between the number of inflamed or painful joints and body composition evaluated by the BMI were obtained by post hoc analysis. This constitutes another significant restriction on internal validity for the results shown in this study.
ConclusionsWe find that being overweight or obese according to the criteria of Stavropoulos-Kalinoglou et al. in patients with RA is associated with a higher number of inflamed joints. It is also significantly correlated with the number of inflamed joints and the score of the majority of anthropometric body composition measurements, such as BMI, arm and waist circumference, triceps fold and the percentage of body fat evaluated by BEI and plicometry. As a whole, these results support the hypothesis that the level of inflammatory activity is modulated by the volume of body fat in patients with RA. Finally, we believe our results support the need for clinical trials to evaluate the effect of body composition correction or optimisation on the clinical condition of patients with RA. If this intervention is proven to have an effect, monitoring nutritional status may come to occupy a central role in the basic recommendations for the management of RA.
FinancingThe resources used to carry out this study originate in the institution where it took place.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Alvarez-Nemegyei J, Pacheco-Pantoja E, González-Salazar M, López-Villanueva RF, May-Kim S, Martínez-Vargas L, et al. Asociación entre sobrepeso/obesidad y estado clínico en artritis reumatoide. Reumatol Clin. 2020;16:462–467.