To determinate the diagnostic value of an antibody against a citrullinated fibrinogen peptide in Cuban patients with rheumatoid arthritis, using an enzyme immunoassay.
Materials and methodsA citrullinated peptide of fibrinogen designed by informatics prediction was synthesised and used in an enzyme immunoassay. The participants were 81 patients with early disease, 81 patients with established disease, 58 patients with other rheumatic and inflammatory diseases, and 43 healthy individuals. Anti-citrullinated fibrinogen peptide, anti-mutated citrullinated vimentin, anti second generation citrullinated peptides and rheumatoid factor antibodies were determined by enzyme-linked immunosorbent assay.
ResultsDetermination of anti-citrullinated peptide of fibrinogen antibodies by the designed enzyme immunoassay showed the best diagnostic value in early rheumatoid arthritis patients, with the highest value sensitivity (84%), negative predictive value (85%), Youden index (0.73%) and area under the receiver operating curve (0.9192). Specificity (89%) and positive predictive value (88%) were higher than rheumatoid factor, similar to anti-mutated citrullinated vimentin, but lower than second generation anti-citrullinated peptides assay. The positivity of C-reactive protein was associated with the presence of anti-citrullinated fibrinogen peptide antibodies and the titres of these antibodies correlated with clinical activity in early disease.
ConclusionsThe immunoassay designed with a citrullinated fibrinogen peptide has a high diagnostic value and can identify patients with greater clinical activity in early rheumatoid arthritis.
Determinar el valor diagnóstico de los anticuerpos contra un péptido del fibrinógeno citrulinado, en pacientes cubanos con artritis reumatoide (AR), mediante un inmunoanálisis enzimático.
Materiales y métodosSe sintetizó un péptido del fibrinógeno citrulinado diseñado por predicción informática y se utilizó en un inmunoanálisis enzimático. Participaron 81 pacientes con AR temprana, 81 pacientes con AR establecida, 58 pacientes con otras enfermedades reumáticas e inflamatorias y 43 individuos sanos. Se determinaron los anticuerpos contra el péptido citrulinado del fibrinógeno, antivimentina citrulinada mutada, antipéptidos citrulinados de segunda generación y factor reumatoide mediante el ensayo de inmunoabsorción ligaado a enzimas (ELISA).
ResultadosLa determinación de anticuerpos contra el péptido citrulinado del fibrinógeno mediante el inmunoanálisis enzimático diseñado mostró el mejor desempeño diagnóstico en pacientes con enfermedad temprana, con el valor más elevado de sensibilidad (84%), valor predictivo negativo (85%), índice de Youden (0,73%) y área bajo la curva operativa del receptor (0,9192). La especificidad (89%) y el valor predictivo positivo (88%) fueron superiores al factor reumatoide, similar al ensayo antivimentina citrulinada mutada, pero inferiores al ensayo antipéptidos citrulinados de segunda generación. La positividad de proteína C reactiva se asoció a la presencia de anticuerpos contra el péptido citrulinado del fibrinógeno y los títulos de estos anticuerpos tuvieron correlación con la actividad clínica en la enfermedad temprana.
ConclusionesEl inmunoanálisis diseñado con un péptido citrulinado del fibrinógeno tiene un alto valor diagnóstico y permite la identificación de pacientes con mayor actividad clínica en la AR temprana.
Rheumatoid arthritis (RA) is a disease that is characterised by chronic inflammation and the presence of auto-antibodies. It affects the small peripheral joints with a high degree of morbidity and disability.1,2
Although the aetiopathogenesis of RA has not been completely elucidated, there is evidence that indicates that influenced by complex environmental and genetic interactions, the organisms own antigens with posttranslational modifications such as citrullination are presented to the immune system, leading to breakdown of the processes involved in immunological tolerance.3–6
The disease was diagnosed using the 1987 criteria of the American College of Rheumatology. This sets 6 clinical criteria and rheumatoid factor (RF) as the sole serological criterion.7 As protein citrullination has been implicated in the pathogenic mechanisms of RA, antibodies against citrullinated peptides have been included in the latest criteria of the American College of Rheumatology and the European League against Rheumatism for diagnosis of the disease.8
The first antibodies against citrullinated peptides described were perinuclear factor and anti-keratin antibodies. These react with profilaggrin protein, identified by indirect immunofluorescence.9 The insertion of cyclical changes into lineal filaggrin peptides gave rise to the first generation of assays for the determination of antibodies against citrullinated peptides, which are more sensitive for diagnosis.9
One of the most widely-used assays is the determination of antibodies against second generation citrullinated peptides (anti-CCP2), which as well as being highly specific, are also present in early stages of the disease and are predictive of joint erosion in patients with RA.8,10,11
The third generation of immunoanalysis (anti-CCP3) was designed using peptide engineering. Several authors have shown that there is no major difference between these and the anti-CCP2 assay.11,12 Other authors state that they have higher diagnostic value than anti-CCP2 antibodies.13
On the other hand, citrullinated proteins which are highly specific for diagnosis have been detected in the synovial tissue of the inflamed joints of patients with RA (fibrinogen, vimentin, enolase, fibronectin and type II collagen). These proteins are thought to be relevant candidates for triggering autoimmunity in genetically susceptible individuals.14,15
Our aim in this work was to determine the diagnostic value of antibodies against a citrullinated fibrinogen peptide in Cuban patients with early stage and established RA, by means of enzymatic immunoanalysis.
Material and methodsA transversal study was performed.
PatientsThe sample was composed of 162 patients included consecutively in the study (81 patients with early stage RA diagnosed less than one year beforehand and 81 patients with established RA). They were of both sexes and older than 18 years old. They were treated in the National Centre of Rheumatology of Cuba, and they fulfilled the criteria of the American College of Rheumatology and the European League against Rheumatism.8 Patients with neoplasias and pregnant patients were excluded. 25 healthy individuals from the provincial blood bank of La Habana also took part, together with 56 individuals with other autoimmune or autoinflammatory diseases or chronic infections (13 patients with symptoms but with no definitive diagnosis of RA, 10 with mixed disease of connective tissue, 10 with chronic viral hepatitis, 9 with systemic lupus erythematosus, 4 with scleroderma, 3 with psoriatic arthritis, 3 with spondyloarthropathies, 3 with autoimmune myositis and one with Sjögren's syndrome) from the National Genetic Medicine Centre, Cuba. This study was approved by the Ethics Committee of the National Genetic Medicine Centre and all of the participants gave their informed consent.
Determination of disease activity indicatorsThe erythrocyte sedimentation rate (ESR) was determined in 2ml of blood anticoagulated with 0.5ml sodium citrate (3.8%), in 1h.16 Reactive C protein was determined by the qualitative technique of agglutination in latex (Diagnostic Automation/Cortez Diagnostics, USA), by adding a drop of patient serum in the latex sheets and observing the presence of turbidity in 2min reaction. The health assessment questionnaire (HAQ) was applied and the clinical indicator of disease activity based on the ESR (DAS 28) was calculated. DAS 28 was considered to show remission (DAS 28 <2.6), DAS 28 low activity (DAS 28 ≥2.6 and ≤3.2), moderate activity (DAS 28 >3.2 and ≤5.1) and high activity (DAS 28 >5.1).8,17
Design and obtaining citrullinated fibrinogen peptideAntigenic determinants of the α and β fibrinogen chains were predicted, in the three-dimensional structure of the protein obtained from the RSCB-PDB-101 data bank, using the computer programs BepiPred 1.0 (DTU Bioinformatics, Technical University of Denmark, Lyngby, Denmark)18 and Discotope 1.2 (DTU Bioinformatics).19 2 fibrinogen peptides were selected. The covalent bond was formed of both peptides with an intermediate cysteine amino acid between a peptidic chain of α fibrinogen and a chain of β fibrinogen, with the location of a final cysteine and the substitution of the terminal carboxylic termination by terminal carboxamide. Both peptidic subunits were citrullinated in 2 arginine residues. Solid phase synthesis was performed using Fmoc/tBu (9-fluoerenylmethyloxycarbonyl/tert-butyl) methodology. Purification was carried out using preparative reverse phase high performance liquid chromatography (HPLC-RP) in LabChrom equipment (Merck LaChrom, Hitachi High-Technologies Corporation, Germany). Degree of purity was determined by analytic HPLC-RP in AKTA 100 equipment (GE Healthcare, Groton, CT, USA). Molecular mass determination was performed using electronebulisation ionisation mass spectrometry in a Q-Tof spectrometer (Micromass, Ltd., Manchester, United Kingdom). The peptide was synthesised in its native (uncitrullinated) form to compare reactivity with the citrullinated fibrinogen peptide.
Determination of antibodiesThe determination of IgG type designed antipeptide citrullinated fibrinogen antibodies (anti-CFP) was performed by an indirect type enzyme-linked immunoabsorbent assay (ELISA). 96 well maximum absorption plates were sensitised (Thermo Ficher Scientific, NUNC, Roskilde, Denmark) with fibrinogen peptide at a concentration of 10μg/ml in buffered saline phosphate solution 0.15M, pH=7.2 (PBS). It was incubated for 16h in a humidity chamber at 20–25°C. It was washed 3 times with PBS and Tween 20 at 0.05% in a micro ELISA washer (SUMA, MW-2001 Centro de Inmunoensayos, La Habana, Cuba), adding 200μl in each well. Bovine serum albumin (BSA) was added (SIGMA, Aldrich, St Louis, MO, USA) diluted at 2% in PBS, it was incubated for 1h in a humidity chamber at 20–25°C before being washed again. Positive and negative controls were prepared from patients with RA and healthy individuals, respectively, and in the same way as the dilute samples 1/100 in PBS, BSA 2% and Tween 20 at 0.05% were added to them. Standard serum was added, from 120U/ml to 6.2U/ml, prepared using serum from patients with RA, the concentration of which was evaluated in comparison with standard assay serum anti-CCP2 (IBL, International GmbH, Hamburg, Germany). It was incubated at 20–25°C during 1h in a humidity chamber. After another step of washing the conjugated anti-IgG with peroxidase was added (Dako cytomation, Dako, Glostrup, Denmark) 100μl in each well at the dilution suggested for the reagent (1/6000), in PBS, Tween 20 at 0.05%. It was incubated for 1h at 20–25°C and the plate was washed. The dihydrochloric ortho-phenylendiamine substrate wad added (SIGMA, USA) 1mg/ml diluted in the buffered solution sodium citrate 0.1mol/l, pH 5.5 with hydrogen peroxide 1mg/ml (Merck, Germany). It was incubated for 30min at a temperature of 20±5°C. The reaction was halted by the addition of 50μl sulphuric acid 3mol/l (Merck, Germany) in water, and absorbency was measured at 492nm in an ELISA PR-521 reader (SUMA, Cuba). A logarithmic adjustment was used for the calibration curve. The determination of type IgG anti-CCP2 antibodies was performed by ELISA (IBL International, Germany), with a suggested cut-off point of 30U/ml. The determinations of mutated citrullinated antivimentin antibodies (anti-MCV) and IgM type RF were performed by ELISA (Orgentec, Diagnostika GmbH, Mainz, Germany), with a suggested cut-off point of 20U/ml for each assay.
Statistical analysisThe Statistica 7.0 (StatSoft, Inc., Tulsa, OK, USA) and EPIDAT 3.1 (Dirección General de Salud Pública, Junta de Galicia, PanAmerican Health Organisation PHO/WHO) programs were used. Qualitative variables were expressed as frequencies and percentages. χ2 was calculated with Fisher's exact test for expected values below 5, and the odds ratio (OR) was estimated as a magnitude of association. The differences between the optical densities of the controls were determined by Student's t-test. Quantitative variables with a distribution other than normal were expressed as a median with interquartile ranges. Quantitative variables were compared between groups using the Mann–Whitney U test. Spearman's coefficient was determined for correlation. To determine diagnostic value contingency tables were created for all of the patients with RA, in comparison with the healthy individuals and patients with other autoimmune or autoinflammatory diseases or chronic infections. The same procedure was followed separately for the patients with early stage RA and the group of patients with established RA. Diagnostic sensitivity and specificity were calculated, as well as the positive and negative predictive values. The Youden index (YI) was calculated as was the area under the receiver operating characteristic (ROC) curve. The level of statistical significance used was 0.05.
ResultsThe patients included in early stage RA group had a mean age of 48 years old (39–53 years old). 60 of these patients were recently diagnosed and in 21 patients the median duration of the disease was 7 months (5–12 months) after diagnosis. The median age of the patients with established RA was 51 years old (44–61 years old), with a median duration of the disease of 5 years (2–10 years). There was a high proportion of female patients in the early stage RA group (76.5%) and in the established RA group (82.7%).
The prediction of antigenic determinants with the α and β chains of fibrinogen protein made it possible to obtain different epitopes with probable antigenicity to react with ACPC in patients with RA. The regions with the largest amount of amino acids obtained in the predictions with fibrinogen were amino acid regions from 210 to 232 of the α chain and from 16 to 69 of the β chain (Table 1).
Amino acid regions of the epitopes obtained in the prediction of fibrinogen α and β chains, where both computer programs agree.
| Fibrinogen chain | Computer program | |
|---|---|---|
| BepiPred | Discotop | |
| α fibrinogen | 26–48, 59–61, 78–85, 98–110, 175–184, 194–199, 210–232 | 30–39, 60–68, 70–89, 96–117, 174–177, 193–210 |
| β fibrinogen | 16–69, 91–102, 105–113, 129–141, 143–146, 156–162, 216–222, 230–234, 243–250, 254–288, 304–305, 316–324, 330–337, 345–354, 359–362, 378–402, 408–419, 427–434 | 55, 90–99, 110, 123–142, 144–149, 158–159, 221, 231–234, 243–246, 256–257, 304–306, 315–322, 332–337, 348, 359–364, 377–381, 384–392, 396–397, 417–425, 429–431 |
The sequence of amino acids selected was Lys Asp Ile Ile Pro Ser citrulline Asp Arg Gln His citrulline Pro Leu Asp Lys Lys Arg Glu Glu Cys (KDLLPS citrulline DRQHCGH citrulline PLDKKREEC), covering 1.9% of the α chain of fibrinogen (11/560 amino acids) and 2.4% of the β chain of fibrinogen (11/461). The designed peptide had a net positive charge (2+), an arginine/citrulline balance of 1, a molecular mass of 2916.46±0.02Da, which coincides with the theoretical molecular mass of 2916.46Da, and a purity of 98.3.
When ELISA was performed with the designed citrullinated fibrinogen peptide and the uncitrullinated peptide, no differences were found between the positive and negative controls (P=.6644).
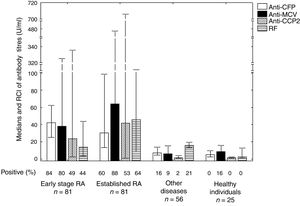
In the patients with early stage RA the antibodies detected the most were anti-CFP antibodies (84.0%) (Fig. 1). The frequency of positivity of all the antibodies studies was higher in the patients with RA than it was in the controls. In the total of patients with RA, titres were higher for anti-CFP antibodies (P=.0000), anti-MCV antibodies (P=.0000), anti-CCP2 antibodies (P=.0000) and RF (P=.0027), in comparison with the total of patients with other diseases and healthy individuals. Differences were observed between the titres of RF antibodies in patients with early stage RA and established RA (P=.0121).
Frequency of antibody positivity in patients with early stage and established RA, in healthy controls and in controls with other diseases.
Differences were found between the titres of antibodies in all of the patients with RA compared to the total of patients with other diseases and healthy individuals. This was so for anti-CFP antibodies (P=.0000), anti-MCV antibodies (P=.0000), anti-CCP2 antibodies (P=.0000) and RF antibodies (P=.0027). Differences were found in the titres of RF antibodies in patients with early stage and established RA (P=.0121).
RA: rheumatoid arthritis; CCP2: second generation citrullinated peptides; RF: rheumatoid factor; MCV: mutated citrullinated vimentin; n: total number of patients; CFP: citrullinated fibrinogen peptide; RCI: interquartile ranges.
The most frequent antibodies in patients who had no anti-CFP antibodies were anti-MCV antibodies in early stage RA (84.6%) and anti-CCP2 antibodies in established (68.8%).
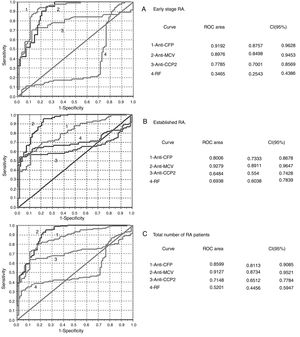
Analysis of YI (Table 2) and the ROC curves (Fig. 2), anti-CFP antibodies performed the best in diagnosing early stage RA.
Diagnostic performance of the antibody determination methods in patients with RA at the cut-off point selected for each method.
| Method | Sensitivity (%); CI | Specificity (%); CI | PPV (%); CI | NPV (%); CI | YI; CI |
|---|---|---|---|---|---|
| Early stage RA (n=81) | |||||
| Anti-CFP | 84.0; 75.3–92.6 | 88.9; 81.4–96.4 | 88.3; 80.5–96.1 | 84.7; 76.5–93.0 | 0.73; 0.62–0.83 |
| Anti-MCV | 80.3; 71.0–89.5 | 88.9; 81.4–96.4 | 87.8; 79.7–96.0 | 81.8; 73.2–90.4 | 0.69; 0.6–0.8 |
| Anti-CCP2 | 49.4; 37.9–60.9 | 98.8; 95.7–100.0 | 97.6; 91.6–100.0 | 66.1; 57.3–75.0 | 0.48; 0.37–0.59 |
| RF | 44.4; 33.0–55.9 | 85.2; 76.8–93.5 | 75.0; 61.7–88.3 | 60.5; 51.1–69.9 | 0.30; 0.2–0.4 |
| Established RA (n=81) | |||||
| Anti-CFP | 60.5; 49.2–71.8 | 88.9; 81.4–96.4 | 84.5; 74.3–94.7 | 69.2. 59.9–78.6 | 0.49; 0.37–0.62 |
| Anti-MCV | 87.7; 79.8–95.4 | 88.9; 81.4–96.4 | 88.9; 81.2–96.3 | 87.8; 80.1–95.5 | 0.77; 0.67–0.86 |
| Anti-CCP2 | 53.1; 41.6–64.6 | 98.8; 95.7–100.0 | 97.7 92.2–100.0 | 67.8 58.9–76.7 | 0.52 0.41–0.63 |
| RF | 64.2; 53.1–75.3 | 85.2; 76.8–93.5 | 81.3; 70.9–91.6 | 70.4; 60.9–80.0 | 0.49; 0.36–0.62 |
| Total RA (n=162) | |||||
| Anti-CFP | 72.2; 65.0–79.4 | 88.9; 81.4–96.4 | 92.9; 88.0–97.8 | 61.5; 52.3–70.8 | 0.61; 0.51–0.71 |
| Anti-MCV | 84.0; 78.0–89.9 | 88.9; 81.4–96.4 | 93.8; 89.5–98.1 | 73.5; 64.2–82.7 | 0.73; 0.64–0.82 |
| Anti-CCP2 | 51.2; 43.2–59.2 | 98.8; 95.7–100.0 | 98.8; 95.9–100.0 | 50.3; 42.2–58.4 | 0.50; 0.42–0.58 |
| RF | 51.9; 43.9–59.9 | 85.2; 76.8–93.5 | 87.5; 80.4–94.6 | 46.9; 38.5–55.4 | 0.37; 0.26–0.48 |
RA: rheumatoid arthritis; CCP2: second generation citrullinated peptides; RF: rheumatoid factor; CI: confidence interval; YI: Youden index; MCV: mutated citrullinated vimentin; n: number of patients; CFP: citrullinated fibrinogen peptide; CP: cut-off point; NPV: negative predictive value; PPV: positive predictive value.
Analysis of the ROC curves obtained in evaluation of the diagnostic performance of the immunoanalysis. (A) Early stage RA. (B) Established RA. (C) Total number of RA patients.
The area homogeneity test showed differences between all of the curves (P=.000).
RA: rheumatoid arthritis; CCP2: second generation citrullinated peptides; RF: rheumatoid factor; CI: confidence interval; MCV: mutated citrullinated vimentin; CFP: citrullinated fibrinogen peptide; ROC curve: receiver operative curve.
All of the patients with early stage RA had moderate or high DAS 28 scores, as did 80.2% of patients with established RA (Table 3), with a higher median of 5.1. The highest rate of positivity for reactive C protein was observed in patients with early stage RA. Reactive protein C positivity was shown to be associated with the presence of anti-CFP antibodies (χ2=5.8; OR=4.7; CI=1.2–17.9; P=.0163) in early stage RA and established RA (χ2=5.3; OR=2.9; CI=1.2–7.4; P=.0217). Additionally, in early stage RA a positive correlation was observed between anti-CFP antibodies and the DAS 28, as well as higher DAS 28 scores in patients positive for anti-CFP antibodies in comparison with seronegative patients (U=244.0; P=.0108).
Clinical and serological indicators of disease activity in patients with RA and in association with positivity for anti-CFP antibodies.
| Indicators | Early stage RA (n=81) | Established RA (n=81) |
|---|---|---|
| DAS 28, m (RCI) | 5.2 (4.3–6.0) | 5.1 (3.9–5.9) |
| Remission: DAS 28 <2.6, n (%) | 0 | 7 (8.6) |
| Low activity: DAS 28 ≥2.6 and ≤3.2, n (%) | 0 | 9 (11.1) |
| Moderate: DAS 28 >3.2 and ≤5.1, n (%) | 35 (43.2) | 26 (32.1) |
| High: DAS 28 >5.1, n (%) | 43 (53.1) | 39 (48.1) |
| DAS 28 in anti-CFP positives, m (RCI) | 5.4 (4.5–6.2)a | 5.3 (4.8–5.9) |
| DAS 28 in anti-CFP negatives, m (RCI) | 4.5 (3.7–5.2)a | 4.5 (2.9–6.0) |
| HAQ, m (RCI) | 0.62 (0.25–0.75) | 0.75 (0.25–1.31) |
| HAQ in anti-CFP positives, m (RCI) | 0.75 (0.25–0.75) | 0.75 (0.25–1.31) |
| HAQ in anti-CFP negatives, m (RCI) | 0.38 (0.28–0.75) | 0.75 (0.25–1.13) |
| Treatment | ||
| Methotrexate (%) | 25.9 | 81.5 |
| Azathioprine (%) | – | 7.4 |
| Chloroquine (%) | – | 17.3 |
| Sulfasalazine (%) | – | 11.1 |
| Prednisone (%) | 19.8 | 90.1 |
| Positive reactive protein C, n (%) | 68 (84.0) | 48 (59.3) |
| ESR, m (RCI) | 30 (20–48) | 31 (16–55) |
| ESR in anti-CFP positives, m (RCI) | 30 (20–48) | 33 (18–60) |
| ESR in anti-CFP negatives, m (RCI) | 28 (25–40) | 25 (23–45) |
RA: rheumatoid arthritis; DAS 28: disease activity index based on the count of 28 joints; HAQ: health assessment questionnaire; m: median; n: number of patients; CFP: citrullinated fibrinogen peptide; RCI: interquartile range; ESR: erythrocyte sedimentation rate.
The most important finding of this study was the capacity of designed citrullinated fibrinogen peptide to recognise the antibodies present in Cuban patients with RA in a high number of cases.
Several studies have analysed the utility of fibrin-derived peptides in the diagnosis of this disease. The designed peptide had similarities to the peptides evaluated previously in these patients, in terms of coincidence with certain amino acid regions, net positive charge and the arginine/citrulline balance.20–22 According to these researchers, a net positive charge and a suitable balance of arginine/citrulline residues in the peptides may favour a formation that has greater capacity to bind with the autoantibodies.22
Previous studies have shown that not all citrulline peptides have the same reactivity. This shows the importance of the surrounding non-citrullinated amino acid residues in amplifying antigenicity.22 It has been observed that the amino acid residues which surround the citrulline intervene in the affinity of the peptide for the antibodies, where the amino acids found the most often are glycine, serine, histidine, threonine and glutamine.22–24 In the designed peptide the amino acids serine and histidine are found surrounding the citrulline.
The diagnostic value of anti-CFP antibodies was superior to the commercial immunoanalysis methods used habitually (anti-MCV, anti-CCP2 and RF) in Cuban patients with early stage RA, while in the patients with established RA it was only superior to anti-CCP2 and RF assays. The capacity which the modified antigens present in joints such as fibrinogen to trigger the production of antibodies in the early stages of the disease is therefore important.14,15 Previous studies in Cuban patients with RA have shown that the antibodies against modified vimentin antigens have superior diagnostic value than anti-CCP2 antibodies.25
Immunoanalysis to determine the anti-CFP antibodies evaluated showed superior values of sensitivity and lower values of specificity in the diagnosis of early stage RA in comparison with those obtained by other authors, who evaluated citrullinated peptides against other epitopes of fibrin and fibrinogen proteins.21,22,26 In 2009, Sanmarti et al. found a sensitivity of 72.1% and a specificity of 98% for a peptide in the α chain of citrullinated fibrin (617–631, citrullination 630) bonded to filagrine and a sensitivity of 73.9% in the determination of anti-CCP2 antibodies.21 In 2014, Cornillet et al. evaluated a β chain peptide of citrullinated fibrin (60–74, citrullination 60, 72, 74) that had been reported the previous decade, obtaining a sensitivity (71%) and specificity (95%) similar to those obtained using determination of anti-CCP2 antibodies (74% sensitivity and 95% specificity).26 They demonstrated that these fibrin epitopes are recognised by antibodies against citrullinated fibrinogen, and they also studied the reactivity of an α chain citrullinated fibrin peptide (36–50) combined with a β chain peptide (60–74), finding a sensitivity of 47% when specificity was set to 98% (26). Cabrera-Villalba et al., in 2017, found a frequency of 68.5% for an α citrullinated fibrinogen peptide (617–631, citrullination 630) in patients with RA.27 This is less than the frequency of appearance of the antibodies that recognise the designed peptide in early stage RA.
In the Cuban population there may be different antigenic specificities recognised by other antibodies against citrullinated peptides not detected by anti-CCP2 antibodies. The variations in different populations of the frequency of antibodies to recognise citrullinated proteins may be due to genetic and environmental factors, as well as demographic characteristics, patient selection and disease classification.4,11
The presence of anti-CFP antibodies was associated with greater disease severity, so that they are therefore a useful tool for the clinical evaluation of Cuban patients with RA. These antibodies may it possible to differentiate 2 clearly defined patient populations with different clinical characteristics. In early stage RA, the group of seropositive patients is characterised by greater disease activity, with a higher DAS 28 score than seronegative patients. The treatment objectives and strategies for RA may therefore differ in patients who are seropositive for anti-CFP antibodies.
One limitation of this work was that the association of anti-CFP antibodies with radiological studies was not examined, and this may form a part of future clinical research in Cuban patients with RA.
ConclusionsThe immunoanalysis designed with a new citrullinated fibrinogen peptide is able to detect specific antibodies in Cuban patients with early stage and established RA. It has high diagnostic value and may be used to identify patients with greater disease severity.
FinancingThis research was financed by the Public Health Ministry, La Habana, Cuba.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Martínez Téllez G, Torres Rives B, Gómez Morejón JA, Pérez Garay H, Rodríguez AM, Portal Miranda JÁ. Valor diagnóstico de anticuerpos antipéptido citrulinado del fibrinógeno en la artritis reumatoide. Reumatol Clin. 2020;16:455–461.