To describe the therapeutic management of rheumatoid arthritis (RA), psoriatic arthritis (PsA) and ankylosing spondylitis (AS) in patients initiating treatment with biological agents.
Materials and methodsObservational, retrospective, longitudinal study in 33 Spanish hospitals. Patients with RA, PsA and AS starting treatment with biological agents between September 2009 and August 2010 and a follow-up longer than 3 years were included. Clinical-demographic characteristics, drugs, biological therapy survival, and reasons for discontinuation or switching were analysed.
ResultsFour hundred and sixty-three patients were included (183 RA, 119 PsA and 161 AS), with a mean follow-up of 3.8 years. At the end of follow-up, a high proportion continued with the first biological prescribed (41.0% of RA, 59.7% of PsA and 51.6% of AS), 31.1%, 47.9% and 42.9% of RA, PsA and AS patients requiring dosage adjustments, respectively. There was temporary discontinuation in 8.2%, 8.4% and 15.5% of patients, and a switch of biologic agent was required in 37.7%, 26.1% and 24.2%. Definitive discontinuation occurred in 13.1%, 5.9% and 8.7% of RA, PsA and AS patients, respectively. Mean time to discontinuation or switching was 30.1 months for RA and 35.7 months for PsA and AS.
ConclusionsOur results suggest that, in practice, half of patients with RA and two thirds with PsA or AS maintained the first biological, but with frequent dose adjustments.
Describir el manejo terapéutico de la artritis reumatoide (AR), la artritis psoriásica (Aps) y la espondilitis anquilosante (EA) en pacientes que inician tratamiento con agentes biológicos.
Materiales y métodosEstudio observacional, retrospectivo, longitudinal en 33 hospitales españoles. Se incluyeron pacientes con AR, Aps y EA que iniciaron tratamiento con agentes biológicos entre septiembre de 2009 y agosto de 2010, con un seguimiento de más de 3 años. Se analizaron las características clínico-demográficas, los fármacos, la supervivencia de la terapia biológica y las causas de cambio o interrupción.
ResultadosSe incluyeron 463 pacientes (183 AR, 119 Aps y 161 EA) con un seguimiento medio de 3,8 años. Al final del seguimiento una elevada proporción continuaba con el primer biológico prescrito (41,0% de AR, 59,7% de Aps y 51,6% de EA), precisando ajustes de dosis el 31,1%, 47,9% y 42,9% de pacientes con AR, Aps y EA, respectivamente. Hubo interrupción temporal en el 8,2%, 8,4% y 15,5% de los pacientes y se precisó cambio de biológico en el 37,7%, 26,1% y 24,2%. La interrupción definitiva ocurrió en el 13,1%, 5,9% y 8,7% de pacientes con AR, Aps y EA, respectivamente. El tiempo medio de cambio o interrupción fue de 30,1 meses para la AR y de 35,7 meses para la Aps y la EA.
ConclusionesNuestros resultados sugieren que, en la práctica, la mitad de los pacientes con AR y 2/3 con Aps o EA continúan con el primer biológico, pero con frecuentes ajustes de tratamiento.
Rheumatoid arthritis (RA), psoriatic arthritis (Psa) and ankylosing spondylitis (AS) are the most frequent cause of inflammatory arthropathies. They develop with inflammation, pain and damage in the joints and other organs, reducing the functionality of patients and their quality of life, increasing mortality.1 RA, Psa and AS, as a whole, affect 1% of the general population.2,3 These diseases are an economic burden for society, more so taking into account the increase in life expectancy. The existence of better therapeutic options may affect patients’ quality of life and save health service resources and costs.4–6
The development of biological agents has improved the treatment and prognosis for rheumatic patients, reducing the loss of joint function and pain.7,8
The BIOBADASER 2.0 registry showed that 50% of patients had suspended biological treatment after 4 years.9,10 The patients with Psa or AS continued with the treatment for longer than patients with RA, with adherence rates after 3 years for the first biological therapy of 0.73, 0.76 and 0.65, respectively.11 Other studies, including the DANBIO registry, describe a lack of efficacy, side effects, age, sex and patient preferences, among others, as factors associated with adherence to biological drug regimes in rheumatic diseases.10,12–14 Thus in younger patients the most common cause of suspension is lack of efficacy, while in older patients the most common causes are adverse events (AEv), especially in RA. The questions associated with continued pharmacological adherence and the dosage patterns for biological agents in Spain were analysed beforehand in rheumatic patients treated with etanercept.10,14 Nevertheless, there are no data on the data on the overall therapeutic management of patients with rheumatic diseases treated with biological agents in Spain. This is especially so in relation to dose optimisation and switching between different biological agents. The aim of this study is to analyse the therapeutic management of RA, Psa and AS in patients prescribed biological treatment. This includes the initial and subsequent patterns of treatment, the reasons for switching and the continuance of adherence to biological agents.
Materials and methodsStudy designThis observational, retrospective and longitudinal study was undertaken in 33 public hospitals in 14 Spanish autonomous communities. The list of hospitals and research centres is included in the supplementary files. The clinical histories were reviewed of patients with RA, Psa or AS who started treatment with biological agents from 1 September 2009 to 31 August 2010. The date they started taking their first biological drug was defined as the first prescription for RA, Psa or AS. Longitudinal data were extracted that covered from 36 to 48 months after the date of starting to take their first biological drug. The researchers included 5 patients with RA, Psa and AS in their database, selected consecutively according to date of starting to take the biological drug after September 2009. Due to difficulties with including patients from some hospitals a switch was made to competitive inclusion 6 weeks after the end of the recruitment period in the last hospital included. Data extraction took place from August 2013 to May 2014.
The study protocol was approved by the Ethics Committee of the Hospital Clínic i Provincial, Barcelona. It was classified by the Agencia Española de Medicamentos y Productos Sanitarios as a post-authorisation study with designs other than prospective follow-up (EPA-OD). The study was carried out according to the principles of the Helsinki Declaration.
To minimise selection bias all patients were registered who had started treatment with biological agents in the study period, and afterwards the inclusion and exclusion criteria were applied.
The patients had to fulfil all of the inclusion criteria:
- 1)
At least 18 years old on the date they started taking the first biological.
- 2)
Diagnosed RA, Psa or AS on the date of starting to take the first biological, according to the 1987 classification criteria of the American College of Rheumatology or those of the American College of Rheumatology 2010/the European League against Rheumatism10,13 for RA. Psa was classified according to CASPRA criteria14 and it was defined as the presence of at least 3 inflamed and 3 painful joints, negative rheumatoid factor and the presence of psoriasis with a confirmed lesion of at least 2cm in diameter. The 1984 modified New York criteria were used for AS.15
- 3)
Having started treatment with biological agents from 36 to 48 months prior to inclusion, regardless of whether the treatment was interrupted or changed after the said date.
- 4)
With available clinical histories containing at least the minimum obligatory information for the study (after the visit or the date of starting their first biological treatment until the date of inclusion or death).
- 5)
The signed informed consent of the patient, except in those hospitals where this was not considered necessary.
The exclusion criteria were:
- 1)
Medical or psychological disorders that compromised the capacity of the patient to give their informed consent, according to the judgement of the researcher.
- 2)
Participation in any clinical trial during the study period.
- 3)
Biological treatment for any indication other than RA, Psa or AS.
Demographic data were recorded, including age, sex, race, residency and marital status. The presence of concomitant diseases was recorded based on medical criteria using a preset list (osteoporosis, asthma, chronic obstructive pulmonary disease, angina, congestive heart failure, acute myocardial infarct, neurological disease, cerebrovascular accident, peripheral vascular disease, type 1 diabetes, type 2 diabetes, gastrointestinal disease, anxiety, depression, other psychological diseases, visual deficiency, auditory deficiency, obesity or body mass index >30, hypertension, dyslipidaemia, neoplasia and other diseases). Disease characteristics included the use of concomitant treatments (non-steroid anti-inflammatory drugs [NSAIDs], glucocorticoids and disease modifying anti-rheumatic drugs [DMAD]), disease duration, number of inflamed and painful joints, general evaluation by the patient, erythrocyte sedimentation rate (ESR) and disease activity index for 28 joints (DAS28). Biological treatment data included the active drug, dose and frequency prescribed at first, together with any modification to this prescription during the study period. The minimum information that had to be available for each disease was specified on the date of starting to take the first biological drug and at the least in the follow-up visits when treatment was modified. The minimum necessary information obtained in the previous 30 days for patients with RA or Psa included the number of painful and inflamed joints (counting 28 joints), an acute phase reagent (ESR and C-reactive protein). In patients with AS the BASDAI index was used together with an acute phase reagent. In cases of Psa the PASI and BSA indexes were recorded as well as the joint counts, together with BASDAI when applicable.
Study size and statistical methodsDue to the variability in the previously notified results4,10,14 the sample size for each disease was estimated by supposing a 50% proportion of treatment modification, maximising the necessary sample size. The target sample size was 228 patients for each disease, taking a precision of 6.5% into account, and a level of significance of 0.05.
Patients were included regardless of the duration of their follow-up. Follow-up time was calculated as the number of years between the starting date for their first biological drug and their last follow-up visit.
On the date they started taking their first biological drug at the starting dose of the same was described in comparison with the recommendations contained in the technical data sheet of the product, describing 2 deviations: (a) a gradual reduction of the dose, reducing the size of the same or increasing the length of intervals between doses; and (b) gradually increasing the dose, increasing the size of the same or reducing the length of intervals between doses. The changes found during the follow-up period were also classified in the following categories: (a) change of treatment, patients who terminated treatment with the first biological drug they had been prescribed and started treatment with a different biological drug; (b) interruption during at least 12 months, and then recommencing the treatment with the same biological drug; and (c) termination, patients who terminated treatment with a biological drug and did not start treatment with any other biological drug during at least the following 12 months.
According to the study objective no imputation techniques were used to process missing data. The percentage of patients who required modification of the treatment was analysed, together with the time that passed until the first modification, the reason for the modification, the number of modifications adjusted by the duration of follow-up and the average time between consecutive modifications. Kaplan–Meier survival curves were used to describe the time that passed until cessation of the biological treatment (termination or change of biological drug). The patients without termination or change of treatment were considered to be censored data. Cox regression analysis was used to identify independent prognostic factors for abandonment of biological treatment.
All analysis was undertaken using SAS v9.2.
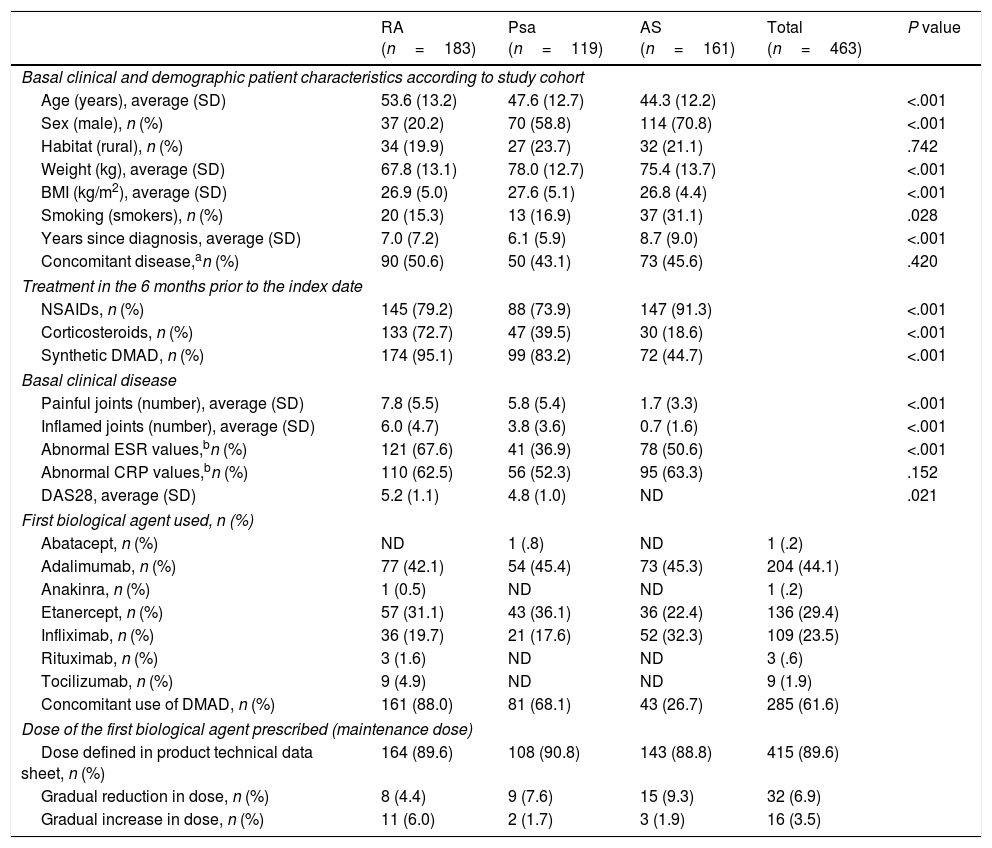
Results492 patients were recruited: 183 (39.5%) with RA, 119 (25.7%) with Psa and 161 (34.8%) with AS; 29 patients were excluded (5.9%) due to the loss of follow-up information or not using a biological drug. At the moment of starting the time since diagnosis was longer in patients with AS than it was for those with RA and Psa. As was expected, fewer patients with AS had previously used synthetic DMAD in the basal period than was the case with patients with RA and Psa. The average DAS28 was lower in patients with Psa than it was for those with RA (Table 1). In the sample with RA, 78.9% of patients were positive for rheumatoid factor, 75.8% were positive for citrullinated antipeptide antibodies and 55.9% had bone erosion. In 68.1% of patients with Psa the disease was mainly located in the peripheral joints, while 10.9% had axial disease and 23.5% had mixed disease. 84.4% of AS patients were HLA-B27 positive.
Basal patient characteristics according to study cohort.
| RA (n=183) | Psa (n=119) | AS (n=161) | Total (n=463) | P value | |
|---|---|---|---|---|---|
| Basal clinical and demographic patient characteristics according to study cohort | |||||
| Age (years), average (SD) | 53.6 (13.2) | 47.6 (12.7) | 44.3 (12.2) | <.001 | |
| Sex (male), n (%) | 37 (20.2) | 70 (58.8) | 114 (70.8) | <.001 | |
| Habitat (rural), n (%) | 34 (19.9) | 27 (23.7) | 32 (21.1) | .742 | |
| Weight (kg), average (SD) | 67.8 (13.1) | 78.0 (12.7) | 75.4 (13.7) | <.001 | |
| BMI (kg/m2), average (SD) | 26.9 (5.0) | 27.6 (5.1) | 26.8 (4.4) | <.001 | |
| Smoking (smokers), n (%) | 20 (15.3) | 13 (16.9) | 37 (31.1) | .028 | |
| Years since diagnosis, average (SD) | 7.0 (7.2) | 6.1 (5.9) | 8.7 (9.0) | <.001 | |
| Concomitant disease,an (%) | 90 (50.6) | 50 (43.1) | 73 (45.6) | .420 | |
| Treatment in the 6 months prior to the index date | |||||
| NSAIDs, n (%) | 145 (79.2) | 88 (73.9) | 147 (91.3) | <.001 | |
| Corticosteroids, n (%) | 133 (72.7) | 47 (39.5) | 30 (18.6) | <.001 | |
| Synthetic DMAD, n (%) | 174 (95.1) | 99 (83.2) | 72 (44.7) | <.001 | |
| Basal clinical disease | |||||
| Painful joints (number), average (SD) | 7.8 (5.5) | 5.8 (5.4) | 1.7 (3.3) | <.001 | |
| Inflamed joints (number), average (SD) | 6.0 (4.7) | 3.8 (3.6) | 0.7 (1.6) | <.001 | |
| Abnormal ESR values,bn (%) | 121 (67.6) | 41 (36.9) | 78 (50.6) | <.001 | |
| Abnormal CRP values,bn (%) | 110 (62.5) | 56 (52.3) | 95 (63.3) | .152 | |
| DAS28, average (SD) | 5.2 (1.1) | 4.8 (1.0) | ND | .021 | |
| First biological agent used, n (%) | |||||
| Abatacept, n (%) | ND | 1 (.8) | ND | 1 (.2) | |
| Adalimumab, n (%) | 77 (42.1) | 54 (45.4) | 73 (45.3) | 204 (44.1) | |
| Anakinra, n (%) | 1 (0.5) | ND | ND | 1 (.2) | |
| Etanercept, n (%) | 57 (31.1) | 43 (36.1) | 36 (22.4) | 136 (29.4) | |
| Infliximab, n (%) | 36 (19.7) | 21 (17.6) | 52 (32.3) | 109 (23.5) | |
| Rituximab, n (%) | 3 (1.6) | ND | ND | 3 (.6) | |
| Tocilizumab, n (%) | 9 (4.9) | ND | ND | 9 (1.9) | |
| Concomitant use of DMAD, n (%) | 161 (88.0) | 81 (68.1) | 43 (26.7) | 285 (61.6) | |
| Dose of the first biological agent prescribed (maintenance dose) | |||||
| Dose defined in product technical data sheet, n (%) | 164 (89.6) | 108 (90.8) | 143 (88.8) | 415 (89.6) | |
| Gradual reduction in dose, n (%) | 8 (4.4) | 9 (7.6) | 15 (9.3) | 32 (6.9) | |
| Gradual increase in dose, n (%) | 11 (6.0) | 2 (1.7) | 3 (1.9) | 16 (3.5) | |
The presence of concomitant diseases was evaluated using a preset list (osteoporosis, asthma, COPD, angina, congestive heart failure, acute myocardial infarct, neurological disease, cerebrovascular accident, peripheral vascular disease, type 1 diabetes, type 2 diabetes, gastrointestinal disease, anxiety, depression, other psychological diseases, visual disability, auditory impediments, obesity or BMI >30, hypertension, dyslipidaemia, neoplasia and other diseases).
According to the reference values used in the laboratories of the participating hospitals.
NSAIDs: non-steroid anti-inflammatory drugs; Psa: psoriatic arthritis; RA: rheumatoid arthritis; DAS28: disease activity index in 28 joints; SD: standard deviation; AS: ankylosing spondylitis; DMAD: disease modifying antirheumatic drug; BMI: body mass index; ND: not defined; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
The chief reason for starting biological treatment was lack of response to the previous treatment (96.7% of patients with RA, 95.8% of those with Psa and 95.7% of those with AS). Adalimumab, etanercept and infliximab were the drugs used the most often, in 204 (44.1%), 136 (29.4%) and 109 (23.5%) patients, respectively (Table 1). The majority of patients started in the maintenance phase of biological treatment using the dose set in the technical data sheet, after which 6.9% gradually reduced the dose and 3.5% raised it.
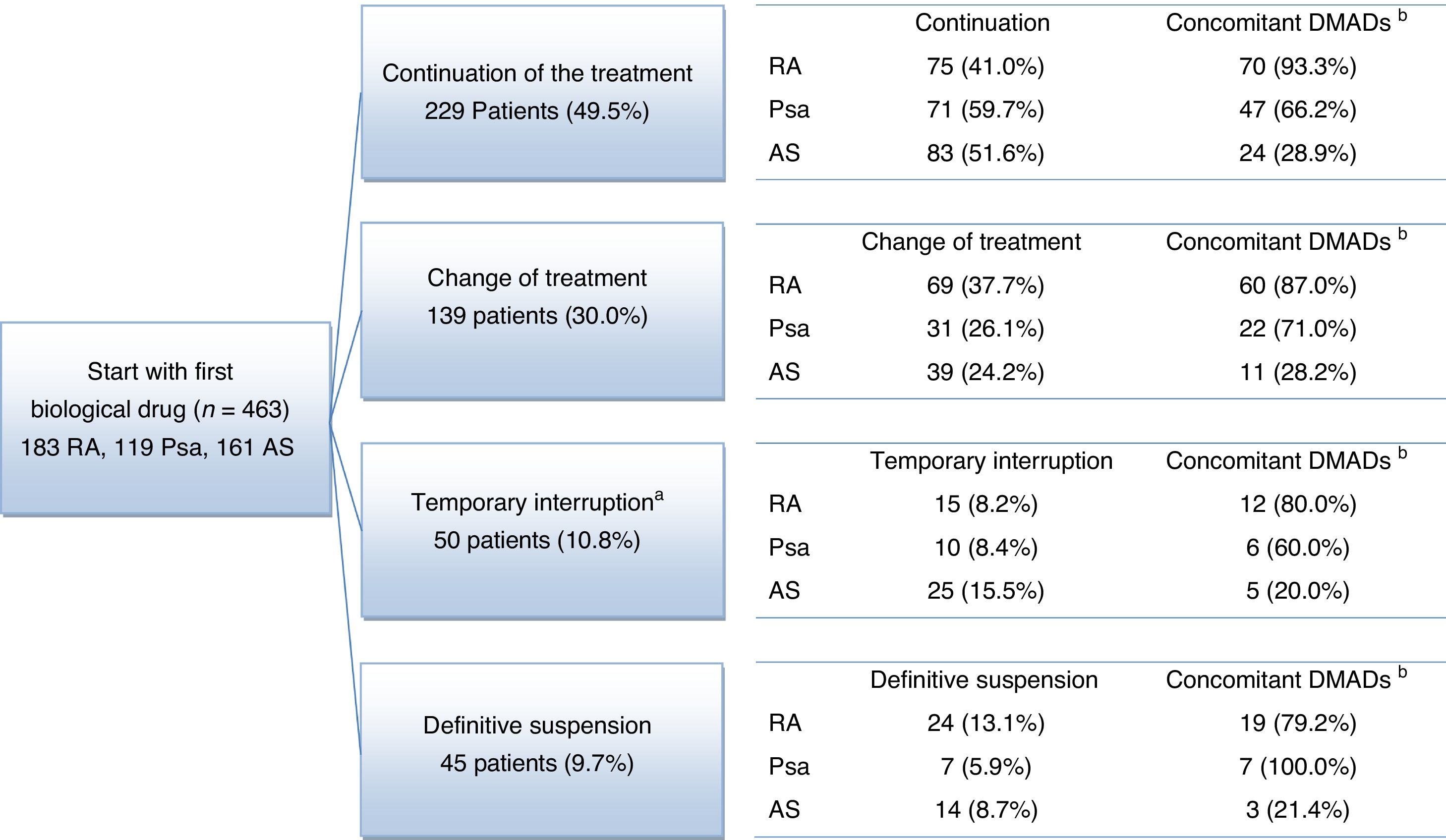
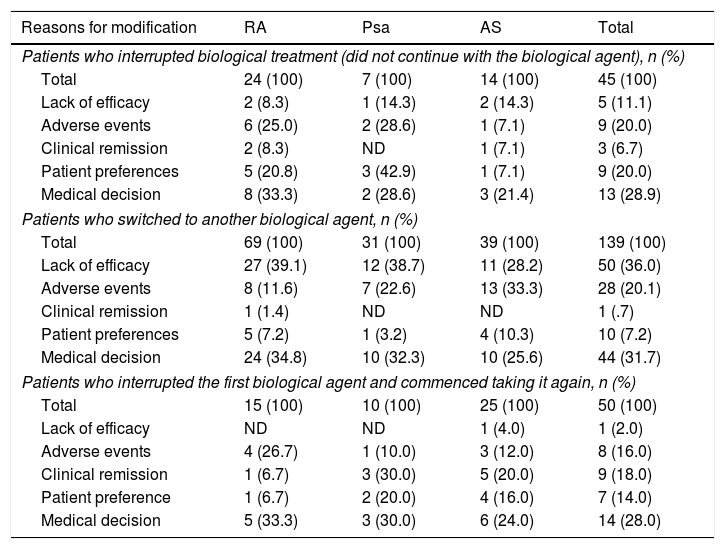
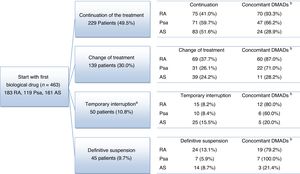
The average follow-up lasted for 3.8 years, being >3 years in 94.8% of patients. At the end of the follow-up period a high proportion of patients continued taking the first biological drug they had been prescribed without interruptions: 75 (41.0%) patients with RA, 71 (59.7%) patients with Psa and 83 (51.6%) patients with AS (Fig. 1). A high percentage of patients also switched from the biological drug they had first been prescribed: 69 (37.7%) patients with RA, 31 (26.1%) patients with Psa and 39 (24.2%) patients with AS. 39 (21.3%) patients with RA, 17 (14.3%) patients with Psa and 39 (24.2%) patients with AS terminated their treatments temporarily or definitively (Fig. 1). The causes reported the most often for terminating or modifying the initial treatment were: the doctor's decision (30.3%), no response (23.9%) and AEv (19.2%) (Table 2).
Flow diagram of rheumatic disease patients.
aThe temporary interruption lasted for up to 12 months in 38 patients (8.2%) and for more than 12 months in 12 patients (2.6%).
bConcomitant: number and percentage of patients who receive at least one concomitant treatment for the diseases studied (NSAID, glucocorticoid and DMAD).
NSAID: non-steroid anti-inflammatory drug; Psa: psoriatic arthritis; RA: rheumatoid arthritis; AS: ankylosing spondylitis; DMAD: disease modifying antirheumatic drug.
Reasons for modifying the initial therapy.
| Reasons for modification | RA | Psa | AS | Total |
|---|---|---|---|---|
| Patients who interrupted biological treatment (did not continue with the biological agent), n (%) | ||||
| Total | 24 (100) | 7 (100) | 14 (100) | 45 (100) |
| Lack of efficacy | 2 (8.3) | 1 (14.3) | 2 (14.3) | 5 (11.1) |
| Adverse events | 6 (25.0) | 2 (28.6) | 1 (7.1) | 9 (20.0) |
| Clinical remission | 2 (8.3) | ND | 1 (7.1) | 3 (6.7) |
| Patient preferences | 5 (20.8) | 3 (42.9) | 1 (7.1) | 9 (20.0) |
| Medical decision | 8 (33.3) | 2 (28.6) | 3 (21.4) | 13 (28.9) |
| Patients who switched to another biological agent, n (%) | ||||
| Total | 69 (100) | 31 (100) | 39 (100) | 139 (100) |
| Lack of efficacy | 27 (39.1) | 12 (38.7) | 11 (28.2) | 50 (36.0) |
| Adverse events | 8 (11.6) | 7 (22.6) | 13 (33.3) | 28 (20.1) |
| Clinical remission | 1 (1.4) | ND | ND | 1 (.7) |
| Patient preferences | 5 (7.2) | 1 (3.2) | 4 (10.3) | 10 (7.2) |
| Medical decision | 24 (34.8) | 10 (32.3) | 10 (25.6) | 44 (31.7) |
| Patients who interrupted the first biological agent and commenced taking it again, n (%) | ||||
| Total | 15 (100) | 10 (100) | 25 (100) | 50 (100) |
| Lack of efficacy | ND | ND | 1 (4.0) | 1 (2.0) |
| Adverse events | 4 (26.7) | 1 (10.0) | 3 (12.0) | 8 (16.0) |
| Clinical remission | 1 (6.7) | 3 (30.0) | 5 (20.0) | 9 (18.0) |
| Patient preference | 1 (6.7) | 2 (20.0) | 4 (16.0) | 7 (14.0) |
| Medical decision | 5 (33.3) | 3 (30.0) | 6 (24.0) | 14 (28.0) |
aMore than one reason for modification is permitted.
Psa: psoriatic arthritis; RA: rheumatoid arthritis; AS: ankylosing spondylitis; ND: not defined.
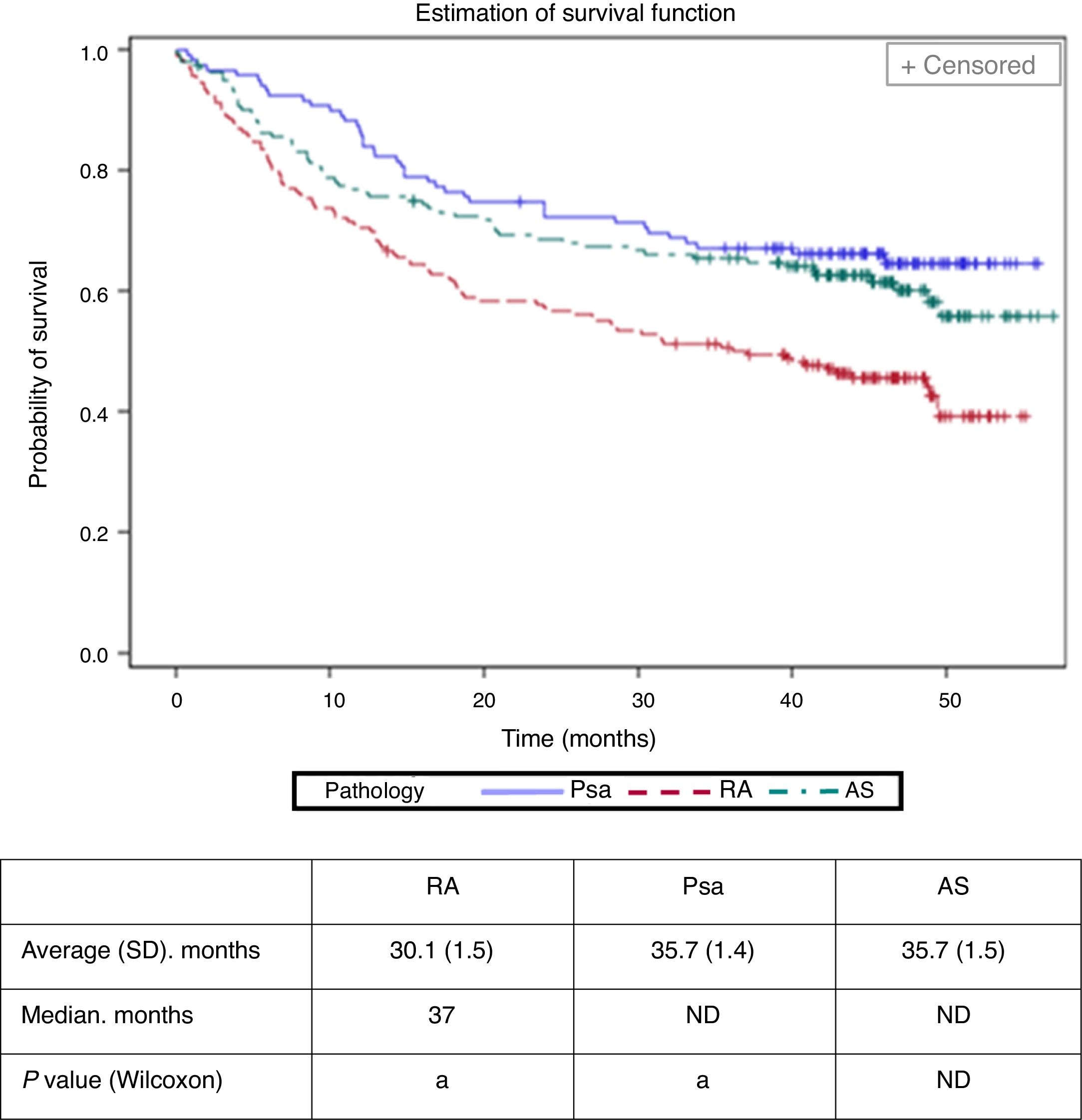
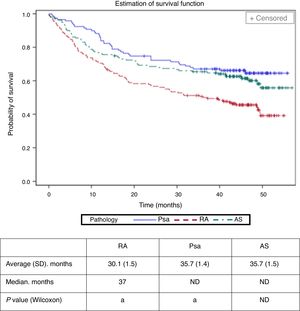
The average time (standard deviation, SD) until abandonment of a biological treatment (termination or change of biological agent) was calculated to stand at 30.1 (1.5) months in RA, 35.7 (1.4) months in Psa and at 35.7 (1.5) months in AS (Fig. 2). It was less in patients with RA (Bonferroni P value <.01). Cox's regression model identified RA (hazard ratio: 1.77; CI 95%: 1.22–2.55), age > 40 (1.42; 1.01–1.99) and the analogue visual scale of evaluation by the doctor >6 (1.80; 1.17–2.76) as the basal factors associated with the risk of abandoning the first biological drug.
Withdrawal time (change of biological agent or interruption) of the first biological treatment according to disease.
aThe study cohorts with the same letter showed statistically significant differences (P<.01). The average values for Psa and AS were not calculated due to the limited follow-up time.
Psa: psoriatic arthritis; RA: rheumatoid arthritis; SD: standard deviation; AS: ankylosing spondylitis; ND: not defined.
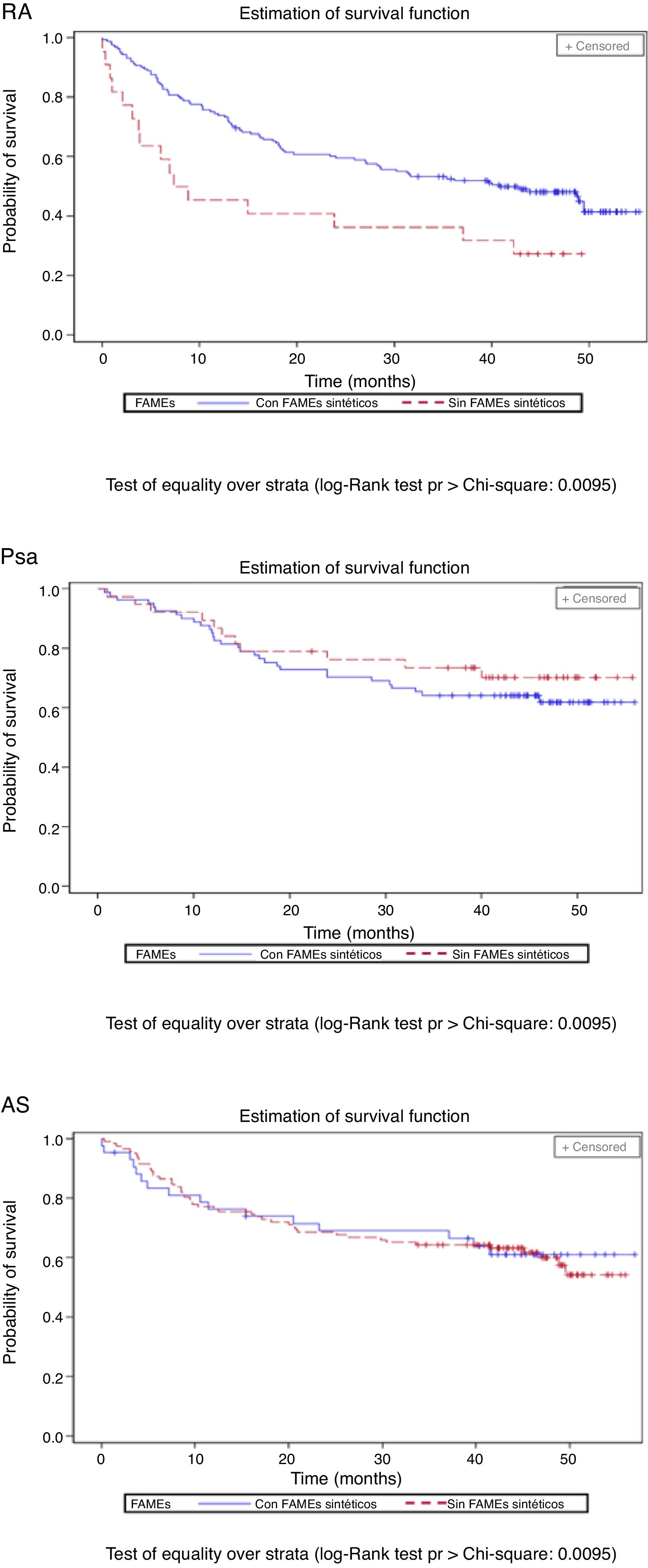
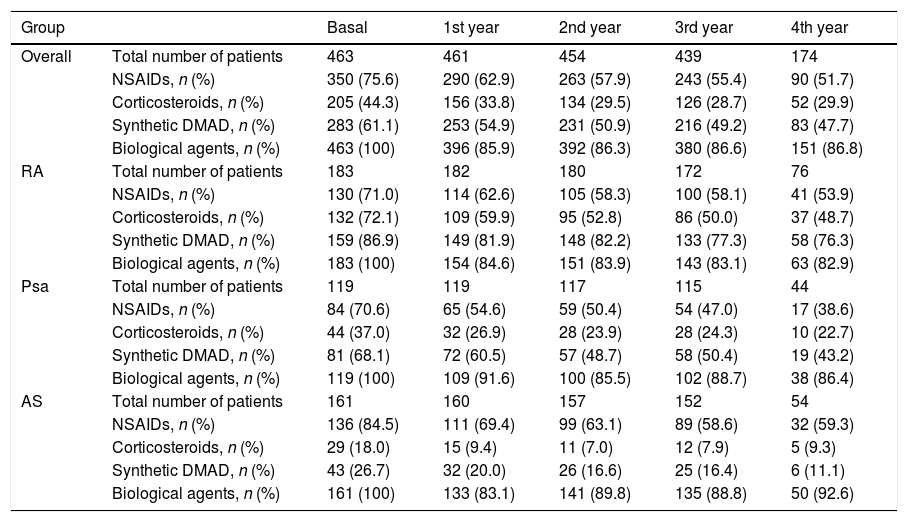
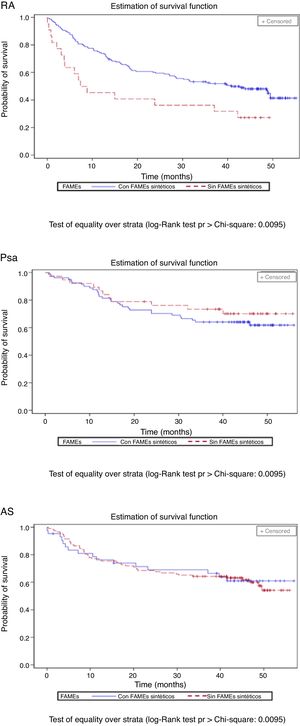
The percentage of patients with concomitant treatments fell during the follow-up period: 23.9% of patients with NSAIDs, 14.4% of those with glucocorticoids and 13.4% of those taking synthetic DMAD (Table 3). Fig. 3 shows the length of time the first biological drug was taken according to the concomitant use of synthetic DMAD. The patients with RA and concomitant treatment with synthetic DMAD and a biological drug displayed a higher retention rate for their first biological drug than those who had not received synthetic DMAD.
Percentage of patients who received concomitant treatment for disease during the follow-up period.
| Group | Basal | 1st year | 2nd year | 3rd year | 4th year | |
|---|---|---|---|---|---|---|
| Overall | Total number of patients | 463 | 461 | 454 | 439 | 174 |
| NSAIDs, n (%) | 350 (75.6) | 290 (62.9) | 263 (57.9) | 243 (55.4) | 90 (51.7) | |
| Corticosteroids, n (%) | 205 (44.3) | 156 (33.8) | 134 (29.5) | 126 (28.7) | 52 (29.9) | |
| Synthetic DMAD, n (%) | 283 (61.1) | 253 (54.9) | 231 (50.9) | 216 (49.2) | 83 (47.7) | |
| Biological agents, n (%) | 463 (100) | 396 (85.9) | 392 (86.3) | 380 (86.6) | 151 (86.8) | |
| RA | Total number of patients | 183 | 182 | 180 | 172 | 76 |
| NSAIDs, n (%) | 130 (71.0) | 114 (62.6) | 105 (58.3) | 100 (58.1) | 41 (53.9) | |
| Corticosteroids, n (%) | 132 (72.1) | 109 (59.9) | 95 (52.8) | 86 (50.0) | 37 (48.7) | |
| Synthetic DMAD, n (%) | 159 (86.9) | 149 (81.9) | 148 (82.2) | 133 (77.3) | 58 (76.3) | |
| Biological agents, n (%) | 183 (100) | 154 (84.6) | 151 (83.9) | 143 (83.1) | 63 (82.9) | |
| Psa | Total number of patients | 119 | 119 | 117 | 115 | 44 |
| NSAIDs, n (%) | 84 (70.6) | 65 (54.6) | 59 (50.4) | 54 (47.0) | 17 (38.6) | |
| Corticosteroids, n (%) | 44 (37.0) | 32 (26.9) | 28 (23.9) | 28 (24.3) | 10 (22.7) | |
| Synthetic DMAD, n (%) | 81 (68.1) | 72 (60.5) | 57 (48.7) | 58 (50.4) | 19 (43.2) | |
| Biological agents, n (%) | 119 (100) | 109 (91.6) | 100 (85.5) | 102 (88.7) | 38 (86.4) | |
| AS | Total number of patients | 161 | 160 | 157 | 152 | 54 |
| NSAIDs, n (%) | 136 (84.5) | 111 (69.4) | 99 (63.1) | 89 (58.6) | 32 (59.3) | |
| Corticosteroids, n (%) | 29 (18.0) | 15 (9.4) | 11 (7.0) | 12 (7.9) | 5 (9.3) | |
| Synthetic DMAD, n (%) | 43 (26.7) | 32 (20.0) | 26 (16.6) | 25 (16.4) | 6 (11.1) | |
| Biological agents, n (%) | 161 (100) | 133 (83.1) | 141 (89.8) | 135 (88.8) | 50 (92.6) |
NSAIDs: non-steroid anti-inflammatory drugs; Psa: psoriatic arthritis; RA: rheumatoid arthritis; AS: ankylosing spondylitis; DMAD: disease modifying antirheumatic drug.
During the follow-up period the patients commenced taking an average of 0.7biologicalagents/year. A total of 5213 medical visits were recorded, with an average of 3.0visits/patient/year, 96.9% of which were by appointment. Three (0.6%) patients died during the follow-up period, although no death was connected with a biological treatment.
Considering only the patients who abandoned, the average duration (SD) of treatment with the first biological drug was 15.0 (13.6) months in patients with RA, 16.1 (11.0) months in patients with Psa and 14.8 (13.7) months in patients with AS. During the follow-up period 159 patients started treatment with a second biological drug, 91 (57.2%) of whom continued with this treatment to the end of the follow-up, while 68 (42.8%) abandoned (terminating or changing to another biological drug). Abandonment of the second biological drug varied according to disease: 38 (48.7%) of 78 patients with RA, 20 (58.8%) of 34 patients with Psa and 10 (21.3%) of 47 patients with AS.
DiscussionThis study describes the pattern of treatment of 3 chronic inflammatory diseases of the joints using biological agents in a large cohort of patients. The study sample is representative of clinical practice in Spain, as it includes patients in 33 hospitals and 14 autonomous communities.
Our cohorts of patients with RA, Psa and AS were followed up over 4 years to describe the pattern of treatment using biological agents. At the end of follow-up 41% of the patients with RA, 59% of those with Psa and 51% of those with AS continued taking the first biological drug they had been prescribed. However, 49%, 69% and 67% of these patients, respectively, had temporarily discontinued taking the drug (during up to 12 months in 8.2% of these patients and during more than 12 months in 2.6% of patients). These results agree with those reported by the BIOBADASER 2.0 registry, where approximately 50% of patients with RA, Psa and AS continued with their initial treatment after 4 years of follow-up.9,10
There are very few data on adjusting the dose of biological agents in clinical practice. Our study shows that adjustment took place in an unexpectedly high percentage of patients: 31%, 48% and 43% of those with RA, Psa and AS, respectively. This adjustment was mainly based on a gradual reduction in the dose, with significantly higher percentages in patients with Psa (43%) and AS (33%), in comparison with RA (24%). This is probably because biological drug achieve better control of the disease in cases of spondyloarthritis.16
The first biological drug prescribed was taken for an average that varied from 30 to 36 months, depending on the disease. This is slightly less than the 43–53 months reported in a study of patients treated with etanercept.14
In our study, the treatment was taken for longer by patients with Psa or AS than it was by those with RA, and this agrees with other published results,9–11 which may be due to the different physiopathologies of RA (which is associated more with T and B cells) and Psa (which is more closely associated with pro-inflammatory cytokines). On the contrary, Senabre-Gallego et al.14 found no differences in the duration of treatment depending on the disease.
The main reasons why patients switched to a second biological drug were lack of response (67%) and AEv (23%). These were also the main reasons for terminating biological treatment reported by 2 Spanish groups,11,12 in which termination due to lack of efficacy was more frequent in younger patients, while it occurred more often due to AEv in older patients.10 Although in our study clinical remission was identified as one of the main reasons for temporary discontinuation, the number of patients with temporary discontinuation was too small for results to be extrapolated.
Our study has several limitations: (1) the use of retrospective data prevents establishing homogeneous definitions and criteria for the hospitals taking part; (2) the availability of biological agents for use on the date of starting to take the first biological drug, as golimumab and certolizumab were not available and there was little experience in Spain with rituximab; and (3) the descriptive aim of the study and the high proportion of patients with censored data makes it impossible to establish the factors associated with discontinuation of the biological treatment.
Nevertheless, the study has the advantages of viability, immediacy and lower risk of introducing bias in clinical practice, and it may help to generate hypotheses for future investigations. In spite of the said limitations, this study made it possible to obtain data gathered in clinical practice that correspond to a larger sample of patients with 3 different diseases over a longer time period.
ConclusionsTo summarise, this observational study shows that the majority of patients with Psa, AS and, to a lesser degree, those with RA, continue taking the first biological drug they were prescribed for a long period of time. The data reflect clinical practice in 33 hospitals distributed throughout Spain. Adjustments to the dose, chiefly in the form of a gradual reduction in the same, and changes of treatment take place in a significant percentage of patients for several reasons, mainly due to doctors’ decisions, efficacy and safety. These real world data may be of interest to understand the duration of biological drug use in the treatment of RA and spondyloarthro-arthritis in clinical practice.
FinancingThis work was financed by Merck Sharp & Dohme (MSD), Spain. MSD took part in the design and execution of the study.
Conflict of interestsJuan D. Cañete was a consultant and/or participated as a speaker for Abbvie, Boehringer, BMS, Celgene, Janssen, Lilly, MSD, Novartis, Novo-Nordisk, Pfizer and UCB.
Antonio Naranjo was a consultant and/or participated as a speaker for Abbvie, Amgen, Gebro, Janssen, Pfizer and Roche, and he took part in projects financed by MSD.
Javier Calvo took part in meetings and studies/clinical trials promoted by Abbvie, Celgene, Lilly, MSD, Novartis, Pfizer and Roche.
Carmen Ordás took part in meetings and studies/clinical trials promoted by Abbvie, BMS, Celgene, Janssen, MSD, Pfizer and Roche.
Belén Aragón and Gonzalo Nocea are MSD employees.
Montse Roset is an employee of IQVIA, which has received fees from MSD for preparing the manuscript and statistical analysis.
Antonio Fernández-Nebro has received fees for consultancy from companies whose products are described in this manuscript or which commercialise products for the same indication (MSD, Abbvie, UCB, Pfizer, Roche and BMS).
The authors would like to thank the researchers who took part in data gathering. See Appendix B (additional material) for the complete list of researchers and hospitals which took part in the study.
Please cite this article as: Cañete JD, Naranjo A, Calvo J, Ordás C, Aragón B, Nocea G, et al. Patrones de tratamiento biológico en pacientes con enfermedades articulares inflamatorias. Estudio retrospectivo de 4 años de seguimiento. Reumatol Clin. 2020;16:447–454.