To evaluate the association of shared epitope, smoking and their interaction on the presence of autoantibodies (anti-cyclic citrullinated peptide [CCP] antibodies and rheumatoid factor) in patients with rheumatoid arthritis in our geographical area.
MethodsA descriptive and cross-sectional study was carried out in a cohort of 106 patients diagnosed with RA. Odds ratios (OR) for antibody development were calculated for shared epitope, tobacco exposure and smoking dose. Statistical analysis was performed with univariate and multivariate statistics using ordinal logistic regression. Odds ratios were calculated with 95% confidence interval (95% CI) and a value of P<.05 was considered significant.
ResultsIn univariate analysis, shared epitope (OR=2.68; 95% CI: 1.11–6.46), tobacco exposure (OR=2.79; 95% CI: 1.12–6.97) and heavy smoker (>20packs/year) (OR=8.93; 95% CI: 1.95–40.82) were associated with the presence of anti-CCP antibodies. For rheumatoid factor, the association was only significant for tobacco exposure (OR=3.89; 95% CI: 1.06–14.28) and smoking dose (OR=8.33; 95% CI: 1.05–66.22). By ordinal logistic regression analysis, an association with high titres of anti-CCP (>200U/mL) was identified with South American mestizos, patients with homozygous shared epitope, positive FR and heavy smokers.
ConclusionsBeing a South American mestizo, having a shared epitope, rheumatoid factor positivity and a smoking dose >20packs/year are independent risk factors for the development of rheumatoid arthritis with a high titre of anti-CCP (>200U/mL). In shared epitope-positive rheumatoid arthritis patients, the intensity of smoking is more strongly associated than tobacco exposure with an increased risk of positive anti-CCP.
Evaluar la asociación del epítopo compartido, el tabaquismo y la interacción entre ambos sobre la presencia de autoanticuerpos (antipéptidos cíclicos citrulinados [anti-PCC] y factor reumatoide) en pacientes con artritis reumatoide en nuestra área geográfica.
MétodosEstudio descriptivo y transversal realizado en una cohorte de 106 pacientes diagnosticados de artritis reumatoide. Análisis estadístico univariante y multivariante mediante regresión logística ordinal. Se calcularon odds ratios (OR) con un intervalo de confianza del 95% [IC95%] y se considero significativo un valor de p<0,05.
ResultadosEn el análisis univariante, el epítopo compartido (OR=2,68; IC95% 1,11-6,46), el hábito tabáquico (OR=2,79; IC95% 1,12-6,97) y un índice de tabaquismo en paquetes-año alto (>20 paquetes/año) (OR=8,93; IC95% 1,95-40,82) se asociaron con la presencia de anti-PCC positivos. Para el factor reumatoide, la asociación solo fue significativa con el hábito tabáquico (OR=3,89; IC95% 1,06-14,28) y el índice de tabaquismo (OR=8,33; IC95% 1,05-66,22). Mediante análisis de regresión logística ordinal se identificó asociación con títulos elevados de anti-PCC (>200U/mL) en mestizos latinoamericanos, ser homocigoto para el epítopo compartido, tener factor reumatoide positivo y ser gran fumador.
ConclusionesEl ser mestizo latinoamericano, tener epítopo compartido, factor reumatoide y un índice de tabaquismo >20 paquetes/año son factores de riesgo independientes para el desarrollo de artritis reumatoide con anti-PCC positivos a títulos elevados (>200U/mL). En los pacientes portadores del epítopo compartido, la intensidad del consumo de tabaco se asocia más fuertemente que el hábito de fumar con un riesgo incrementado de anti-PCC positivos, observándose una interacción entre ambos factores.
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterised by chronic inflammation of the joints. Its origin is multifactorial involving the intervention of genetic and environmental factors whose interaction is determinant in the development of the disease.1 The most important genetic factor for it to develop is a sequence of HLA alleles called “shared epitope” (SE).2 In addition, a wide variety of environmental factors influence the genetic base for the disease's autoimmune process to initiate. Smoking is the most important environmental factor associated with increased risk of RA, particularly in individuals who are SE-carriers.3
The discovery of antibodies against proteins and/or cyclic citrullinated peptide (anti-CCP) has amended the hypothesis of the role of SE as a susceptibility factor. It has been proposed that SE alleles predispose exclusively to the development of anti-CCP positive RA. Furthermore, rheumatoid factor (RF) and anti-CCP antibodies frequently occur simultaneously but the association between SE and RF is secondary to the association between SE and anti-CCP antibodies. In recent years a new model has been formulated for the pathogenesis of RA, where SE and anti-CCP antibodies play an essential role, and where genetic and environmental factors intervene, called the “citrullinated SE model”.4
The aim of our study was to evaluate the association of SE, smoking and the interaction between the two on the presence of specific autoantibodies associated with RA.
Materials and methodsPatientsA study performed on a cohort of 106 patients diagnosed with RA in the Early Arthritis Clinics of the University Hospital Virgen Macarena according to the criteria of the American College of Rheumatology (ACR, 1987).5 A serum sample was taken from each patient on diagnosis to determine anti-CCP antibodies and RF, while SE was determined from a whole blood sample.
Testing for autoantibodies and shared epitopeThe anti-CCP levels were quantified by an ELISA assay (QUANTA Lite™ CCP3 IgG, INNOVA Diagnostics, San Diego, U.S.A.), and a cut of point >40U/mL was considered positive. The RF was quantified using an immunoturbidimetric method powered by latex in the ADVIA 2400 analyser (Siemens Healthcare Diagnostics), and a concentration >20IU/mL was used as the cut-off point. A reverse hybridisation technique was used to determine the SE (“HLA-DRB1 Shared Epitope QKRAA/QRRAA/RRRAA” kit by GenID GmbH, Strasberg, Germany) expressed as negative, heterozygous positive and homozygous positive.
The patients’ smoking exposureThe patients’ exposure to smoking was defined with 2 variables: smoking habit (SH) and smoking dose (SD). SH was defined as “smoker” (including “active smokers” and “ex-smokers”) or “non-smokers”. SD is a quantitative epidemiological variable used to establish patients’ cigarette consumption and is defined as the product between the number of cigarettes smoked per day and the “years smoked” divided by the numerical value of 20. It is expressed in packs/year and classifies patients into 3 groups: high SD (>20 packs/year), low SD (1–20packs/year) and negative SD (non-smokers). A cut-off point of more than 20 packs/year was established to define heavy smokers.6
Ethical aspectsThe research project was approved by the Health Ethics and Research Committee of the Virgen Macarena Hospital of Seville, and the patients’ informed consent was obtained. The principles of fairness, confidentiality, respect, non-infringement and other requirements included in the Good Clinical Practice Guidelines were respected at all times. The study was based on usual clinical practice; therefore at no time did this project involve a change in usual clinical behaviour or the treatments to be followed by the patients.
Statistical data analysisThe quantitative variables were expressed as medians (interquartile range), and the qualitative variables as numbers and percentages. The odds ratio (OR) with 95% confidence intervals (95% CI) were calculated to study the contribution of SE and smoking on the presence of autoantibodies in patients with RA. Different binary logistic and ordinal regression models were constructed, where the dependent variable was to have positive/negative anti-CCP antibodies using different cut off points: those of the technique manufacturer (negative 0–40U/mL, weak positive 40–60U/mL and strong positive >60U/mL); and those of the sample median (negative 0–40U/mL, low positive 40–200U/mL and high positive >200U/mL; median of patients with positive anti-CCP: 200 (63–309)U/mL). The independent variables in the models used were those that with biological sense in the univariate analysis showed a P value<.2.
ResultsPatient characteristicsThe cohort of 106 patients was characterised by a predominance of females (72%), and a median age of 53 (45–62) years (Table 1). Sixty-eight patients (64%) were non-smokers, and the remaining 28 (36%) were smokers: 25 active (24%) and 13 ex-smokers (12%). Of the group of 38 smokers; 25% (26 patients) were heavy smokers with an SD >20packs/year, whereas 11% (12 patients) had a low SD (1–20 packs/year). The SD was observed to be higher in the “ex-smokers” compared to the “active smokers” with values of 45 (36–56) and 26 (18–31) packs/year, respectively (P=.008). Anti-CCP antibodies were positive in 65% of the patients, and this percentage rose to 81% for RF, whereas both antibodies were positive simultaneously in 64% of the patients. SE positivity was 73% (77 patients). Of the 77 patients with SE positivity in the RA group, 60 (57%) were heterozygous, and the remaining 17 (16%) were homozygous.
Patient characteristics at baseline visit.
| Parameters | RA (n=106) | SE-positive RA (n=77) | SE-negative RA (n=29) | P value |
|---|---|---|---|---|
| Age (years) | 53 (45–62) | 55 (46–63) | 52 (39–58) | .2 NSc |
| Female gender | 76 (72) | 56 (72) | 20 (69) | .7 NSb |
| Ethnic group | .04b | |||
| European Caucasians | 99 (91) | 68 (88) | 29 (100) | |
| Latin American mestizos | 9 (9) | 9 (12) | 0 (0) | |
| Family history of RA | 22 (21) | 17 (22) | 5 (17) | .5 NSb |
| Smoking habit | .2 NSb | |||
| Non-smoker | 68 (64) | 48 (62) | 20 (69) | |
| Smokera | 38 (36) | 29 (38) | 9 (31) | |
| Ex-smoker | 13 (12) | 12 (16) | 1 (3) | |
| Active smoker | 25 (24) | 17 (22) | 8 (28) | |
| Smoking dose (packs/year) | 30 (18–45) | 30 (18–50) | 30 (5–31) | .2 NSc |
| Smoking dose | .5 NSb | |||
| Negative | 68 (64) | 48 (62) | 20 (69) | |
| Low (1–20 packs/year) | 12 (11) | 8 (10) | 4 (14) | |
| High (>20 packs/year) | 26 (25) | 21 (27) | 5 (17) | |
| Form of onset of symptoms | .3 NSb | |||
| Polyarticular | 70 (69) | 53 (71) | 17 (65) | |
| Oligoarticular | 21 (21) | 13 (17) | 8 (31) | |
| Monoarticular | 8 (8) | 7 (9) | 1 (4) | |
| Polyarthralgia | 2 (2) | 2 (3) | 0 (0) | |
| Early diagnosis (<2 years) | 83 (83) | 61(82) | 22 (85) | .7 NSb |
| Joint erosion | 64 (60) | 45 (58) | 19 (65) | .5 NSb |
| DAS28 | 5.7 (3.9–6.5) | 5.7 (4.1–6.4) | 5.6 (3.5–6.5) | .8 NSc |
| HAQ | 1.3 (.5–2.0) | 1.4 (.6–2.0) | 1.0 (.5–2.1) | .8 NSc |
| Anti-CCP (U/mL) | 116 (11–280) | 177(16–301) | 16 (6–33) | <.0001c |
| Anti-CCP positivity | 69 (65) | 55 (71) | 14 (48) | .03b |
| RF (UI/mL) | 62 (31–178) | 83 (39–224) | 33 (20–59) | <.0001c |
| RF positivity | 86 (81) | 65 (84) | 21 (72) | .1 NSb |
| Anti-PCC and RF positivity | 60 (64) | 50 (65) | 14 (48) | .1 NSb |
RA: rheumatoid arthritis; anti-CCP: anti-cyclic citrullinated peptide antibodies; DAS28: disease activity score based on 28 joints; SE: shared epitope; RF: rheumatoid factor; HAQ: Health Assessment Questionnaire.
Qualitative data expressed as n (%) and quantitative data expressed as medians (interquartile range).
SE was associated with the presence of anti-CCP antibodies with an OR of 2.68 (95% CI: 1.11–6.46): 71% of the patients with SE positivity had anti-CCP positivity compared to the patients with SE negativity, where this percentage was 48% (Table 2). A significant association was observed with heterozygous positive SE with an OR of 2.50 (95% CI: 1–6.24), and marginal statistical significance with homozygous positive SE with an OR of 3.48 (95% CI: .92–13.25). However, for RF no significant association with SE was found (Table 3).
Factors associated with anti-CCP antibodies in patients with rheumatoid arthritis.
| Parameter | Anti-CCP negativity (n=37) | Anti-CCP positivity (n=69) | OR (95% CI) | P valueb |
|---|---|---|---|---|
| Shared epitope | ||||
| Negative | 15 (52) | 14 (48) | 1 | – |
| Positive | 22 (29) | 55 (71) | 2.68 (1.11–6.46) | .03 |
| Heterozygous positive | 18 (30) | 42 (70) | 2.50 (1–6.24) | .04 |
| Homozygous positive | 4 (23) | 13 (77) | 3.48 (0.92–13.25) | .05 |
| Smoking habit | ||||
| Non-smoker | 29 (43) | 39 (57) | 1 | – |
| Smokera | 8 (21) | 30 (79) | 2.79 (1.12–6.97) | .03 |
| Ex-smoker | 2 (15) | 11 (85) | 4.09 (.84–19.88) | .06 |
| Active smoker | 6 (24) | 19 (76) | 2.36 (.84–6.64) | .1 |
| Smoking dose | ||||
| SD negative (non-smoker) | 29 (43) | 39 (57) | 1 | – |
| Low SD (1–20 packs/year) | 6 (50) | 6 (50) | .90 (.20–2.54) | .7 |
| High SD (>20 packs/year) | 2 (8) | 24 (92) | 8.93 (1.95–40.82) | .005 |
| Gender | ||||
| Male | 7 (23) | 23 (77) | 1 | – |
| Female | 30 (40) | 46 (60) | .47 (.17–1.22) | .1 |
| Agec | ||||
| Age<53 years | 21 (40) | 32 (60) | 1 | – |
| Age>53 years | 16 (30) | 37 (70) | 1.52 (.68–3.39) | .3 |
| Ethnicity | ||||
| European Caucasian | 36 (37) | 61 (63) | 1 | |
| Latin American mestizo | 1 (11) | 8 (89) | 4.72 (.57–39.31) | .1 |
| Family history of RA | ||||
| No family history | 30 (36) | 54 (64) | 1 | |
| With a family history | 7 (32) | 15 (68) | 1.19 (.44–3.24) | .7 |
| Time until diagnosis of the disease | ||||
| Time<2 years | 28 (34) | 55 (66) | 1 | |
| Time>2 years | 5 (29) | 12 (71) | 1.22 (.39–3.81) | .7 |
| Rheumatoid factor | ||||
| Negative (<20IU/mL) | 15 (75) | 5 (25) | 1 | |
| Positive (>20IU/mL) | 22 (26) | 64 (74) | 8.73 (2.84–26.80) | <.0001 |
RA: rheumatoid arthritis; anti-CCP: anti-cyclic citrullinated peptide antibodies; SD: smoking dose; OR: odds ratio.
Data expressed as n (%).
Factors associated with rheumatoid factor positivity in patients with rheumatoid arthritis.
| Shared epitope | RF negativity (n=20) | RF positivity (n=86) | OR (95% CI) | P valueb |
|---|---|---|---|---|
| Shared epitope | ||||
| Negative | 8 (28) | 21 (72) | 1 | – |
| Positive | 12 (16) | 65 (84) | 2.06 (.74–5.73) | .1 |
| Heterozygous positive | 10 (17) | 50 (83) | 1.91 (.66–5.50) | .2 |
| Homozygous positive | 2 (12) | 15 (88) | 2.86 (.53–15.41) | .2 |
| Smoking habit | ||||
| Non-smoker | 17 (25) | 51 (75) | 1 | – |
| Smokera | 3 (8) | 35 (92) | 3.89 (1.06–14.28) | .04 |
| Ex-smoker | 1 (8) | 12 (92) | 4 (.48–33.08) | .2 |
| Active smoker | 2 (8) | 23 (92) | 3.83 (.82–17.98) | .1 |
| Smoking dose | ||||
| SD negative (non-smoker) | 17 (25) | 51 (75) | 1 | – |
| Low SD (1–20 packs/year) | 2 (17) | 10 (83) | 1.67 (.33–8.37) | .5 |
| High SD (>20 packs/year) | 1 (4) | 25 (96) | 8.33 (1.05–66.22) | .04 |
| Gender | ||||
| Male | 3 (10) | 27 (90) | 1 | – |
| Female | 17 (22) | 79 (78) | .39 (.10–1.43) | .1 |
| Agec | ||||
| Age<53 years | 10 (19) | 43 (81) | 1 | – |
| Age>53 years | 10 (19) | 43 (81) | 1 (.38–2.65) | 1.0 |
| Ethnicity | ||||
| European Caucasian | 20 (21) | 77 (79) | 1 | – |
| Latin American mestizo | 0 (0) | 9 (100) | .79 (.72–.88) | .1 |
| Family history of RA | ||||
| No family history | 15 (18) | 69 (82) | 1 | – |
| With a family history | 5 (23) | 17 (77) | .74 (.24–2.32) | .6 |
| Time from diagnosis of the disease | ||||
| Time<2 years | 10 (12) | 73 (88) | 1 | – |
| Time>2 years | 6 (35) | 11 (65) | .25 (.08–.83) | .02 |
RA: rheumatoid arthritis; RF: rheumatoid factor; SD: smoking dose; OR: odds ratio.
Data expressed as n (%).
SH was associated with the presence of anti-CCP positivity with an OR of 2.79 (95% CI: 1.12–6.97). Of the smoker patients, 79% had anti-CCP positivity compared with 57% of the non-smokers (Table 2). For RF an OR of 3.89 (95% CI: 1.06–14.28) was obtained, RF being positive in 92% and 75% of the SH positive and negative patients, respectively (Table 3). When the smoker patients were separated into 2 groups “ex-smokers” and “active smokers”, no statistically significant differences were observed with the presence of anti-CCP and RF, respectively.
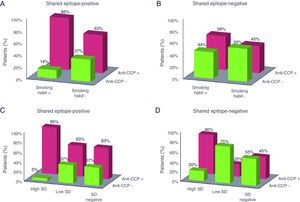
Similarly, we studied in the patients whether the amount of cigarettes smoked influenced the anti-CCP antibodies and RF. A high SD (>20packs/year) was associated with the presence of anti-CCP positivity with an OR of 8.93 (95% CI: 1.95–40.82). Of these, 92% presented anti-CCP positivity compared to the patients with a low SD where the percentages dropped to 50% and 57%, respectively (Table 2). For RF an OR of 8.33 (95% CI: 1.05–66.22) was obtained, RF being positive in 96% and 83% of the patients with a high and low SD, respectively (Table 3). Fig. 1 shows the percentage of patients with anti-CCP positivity and negativity according to SH and SD in the patients diagnosed with RA and stratified according to SE.
Percentage of patients positive and negative for anti-CCP antibodies according to smoking habit and smoking dose of those diagnosed with rheumatoid arthritis and stratified according to shared epitope. The percentage refers to patients with anti-CCP positivity and negativity within each smoking category. Anti-CCP: anti-cyclic citrullinated peptide antibodies; SD: smoking dose. A: shared epitope-positive and smoking habit; B: shared epitope-negative and smoking habit; C: shared epitope-positive and smoking dose; D: shared epitope-negative and smoking dose.
Using an ordinal logistic regression model the association was analysed between risk variables and anti-CCP titres. The best model to explain the association between the presence of negative to positive (0–40U/mL), low positive (40–200U/mL) and high positive (>200U/mL) anti-CCP titres included race, SE, RF and SD. The model was consistent with an OR of 95.94; likelihood ratio of 40.89 and P<.00001. These data are shown in Table 4. The table shows that Latin American mestizos had a proportional OR in the ordinal model of having anti-CCP>200U/mL 14.63 times higher (1.52–141.21) when the remaining variables remain constant. Having homozygous SE increased the OR for having anti-CCP>200U/mL 6.63 (1.88–23.31) times; whereas having heterozygous SE increased the OR of having anti-CCP by half 3.01 (1.22–7.41) if the remaining variables remain constant in the model. Interestingly, having positive RF increased the likelihood of having anti-CCP 5.23 times (1.62–16.85). With regard to SD, being a mild-moderate smoker, i.e., with a SD<20 packs/year was not associated with elevated titres of anti-CCP; however, this association did increase in the heavy smokers with an SD>20 packs/year (OR: 2.64; 95% CI: 1.04–6.69).
Multivariate ordinal logistic regression model of the variables that independently influence the presence of positive anti-CCP antibodies in patients with rheumatoid arthritis.
| Variables | Multivariate model | |
|---|---|---|
| OR (95% CI) | P valuea | |
| Latin American ethnicity | 14.62 (1.52–141.21) | .02 |
| Heterozygous SE | 3.01 (1.22–7.41) | .02 |
| Homozygous SE | 6.63 (1.88–23.31) | .003 |
| Positive RF | 5.23 (1.62–16.85) | .006 |
| Low SD | .55 (.14–2.21) | .4 |
| High SD | 2.64 (1.04–6.69) | .04 |
| Anti-CCP cut-off point 40U/mL | 1.78 (.56–2.99) | – |
| Anti-CCP cut-off point 200U/mL | 3.58 (2.21–4.94) | – |
| Iterations performed to obtain the model: | ||
| Iteration 0: log likelihood=−116.387 | ||
| Iteration 1: log likelihood=−96.467 | ||
| Iteration 2: log likelihood=−95.949 | ||
| Iteration 3: log likelihood=−95.941 | ||
| Iteration 4: log likelihood=−95.941 | ||
| Characteristics of the multivariate model: | ||
| Number of observations: 106 | ||
| LR Chi2=40.89 | ||
| P<.00001 | ||
| Pseudo R2=.1757 | ||
| Log likelihood=−95.94 | ||
| Variables included in the multivariate model: | ||
| Anti-CCP (dependent variable): negative (0–40U/mL), low positive (40–200U/mL), high positive (>200U/mL) | ||
| Ethnicity: Caucasian, Latin American mestizo | ||
| Shared epitope: negative, positive and heterozygous | ||
| Rheumatoid factor: negative (<20IU/mL), positive (>20IU/mL) | ||
| Smoking dose: negative (0 packs/year), low (1–20 packs/year), high (>20 packs/year) | ||
Anti-CCP: anti-cyclic citrullinated peptide antibodies; SE: shared epitope; RF: rheumatoid factor; SD smoking dose; OR: odds ratio.
The ordinal logistic regression model was more consistent than the binary logistic regression model (OR: 95.94 vs 53.44, respectively). The models that included the anti-CCP with a negative (0–40U/mL), weak (40–60U/mL) and strong positive (>60U/mL) cut-off point were less consistent. Data not shown.
DiscussionThis study evaluated the association of SE and smoking on the presence of autoantibodies associated with RA in our southern Spanish population. Most studies on RA have been performed within the European continent, especially in the northern countries, showing consistency in their results.3,7,8 The results obtained have validated previous results in our population and indicate that the presence of SE implies a greater risk of developing RA with positive anti-CCP antibodies (OR=2.68). In addition, the increase in gene dose had an associated increase in risk, coinciding with previous studies. The patients with heterozygous SE had a risk of 2.50, whereas this risk rose to 3.48 for the patients with homozygous SE (Table 2). By contrast, no significant association was observed between SE and the presence of RF. These data show that SE is principally associated with the presence of anti-CCP antibodies, and not the presence of RF in our geographical area.
Without doubt, smoking is the most prominent and contended environmental risk factor in the development of RA. From the first observation at the end of the nineteen eighties of the association of smoking with RA in a group of women,9 consecutive case, control and cohort studies have identified smoking as a major risk factor in RA.1,10,11 Heliövaara et al.12 found that exposure to tobacco smoke can trigger production of RF and contribute to the development of RA, whereas Uhlig et al.13 identified SH as an independent risk factor for developing RA in men, particularly RF-seropositive RA. Likewise, in a study performed in women they found that the duration, but not the intensity, of SH was associated with an increased risk of RA, both RF-seropositive and RF-seronegative.1 However, a study performed by Costenbader et al.11 found that the duration as well as the intensity of the SH were directly related with the development of RA, seropositive RA in particular. In this study the association occurred in both active and ex-smokers.
Therefore, in our patients we also studied the association of smoking with the presence of antibodies (Tables 2 and 3). From the results obtained it was extracted that SH was associated with the presence of anti-CCP antibodies as well as with RF. By contrast, when the smokers were stratified as ex- and active smokers, the results did not become significant. This would support the finding by Costenbader et al.11 who highlight that the association occurs regardless of current tobacco exposure, and affects both active smokers and ex-smokers. SD is a parameter routinely used in patients who are smokers and measures the amount of cigarettes smoked in packs/year and takes into account the number of years that the patient has been smoking. Therefore, in patients with RA we studied the relationship between SD and the presence of the different antibodies. It was found that a high SD (>20packs/year) was associated with the presence of anti-CCP and RF (Tables 2 and 3). However, for a low SD, a consumption of between 1 and 20packs/year, no significant association was obtained with the presence of the antibodies.
Although the aetiology of RA is not entirely known, the findings of recent years indicate that SH acts as an environmental trigger for RA inducing immunity to citrulline residue in SE allele carriers. As described by Klareskog et al.8; SE and smoking do not separately influence the production of autoantibodies but rather interact with each other, and this interaction confers a risk of developing RA but restricted only to what is known as seropositive disease, i.e., to the subgroup of patients with RF-positive RA and/or anti-CCP antibodies but not for RA seronegative to these autoantibodies. The multivariate model obtained in this study by ordinal logistic regression (Table 4) emphasises the importance of the increase in gene dose and intensity of tobacco consumption over the presence of anti-CCP in the patients, supporting previous hypotheses. It is interesting to note, together with these factors, that the patients’ ethnicity and the presence of RF positivity also influence. By contrast, in the patients with seronegative RA no significant association has been described between smoking and SE on the presence of anti-CCP antibodies.3,8,14–16
An interesting finding from the study was that the intensity of smoking quantified by SD was more strongly associated than SH with an increased risk of positive anti-CCP antibodies (Table 2). In fact, the multivariate regression model obtained confirmed this finding including SD but not SH within the independent factors associated with the presence of anti-CCP positivity (Table 4). In the patients with positive SE and high SD, we observed a higher percentage of patients with anti-CCP positivity (95%) compared with the rest of the groups (Fig. 1C). Nevertheless, the high percentage of patients with positive anti-CCP observed in the group of patients with negative SE and high SD (Fig. 1D) is due to the individual effect of accumulated cigarette smoking. Although many studies only assess the interaction between SE and SH and find a significant interaction between the two factors, there are fewer studies that take both SH and SD into account. In one of these studies, Karlson et al.1 found a strong genetic-environmental interaction between SE and smoking when stratifying according to SD rather than SH for the development of seropositive RA. Recently, a study performed in various cohorts of patients with RA found that smoking is not associated with the presence of anti-CCP per se but rather with the concurrent presence of several autoantibodies in RA.17
Anti-CCP antibodies play a fundamental role in the pathogenesis of RA.8,18,19 The first stage of this process involves the induction of anti-CCP antibodies through the generation of citrullinated neoepitopes either due to a local inflammatory event or the action of environmental agents. These proteins and citrullinated peptides would constitute the substrate for activating a local immune response with the production of anti-CCP. This primary stage can take place several years before RA develops. Subsequently, the second stage involving the presence of a joint inflammatory process citrullinisation of peptides and proteins would be triggered in the inflamed joints that would be recognised by the previously generated anti-CCP giving rise to an immune response with release of inflammation mediators such as interleukins and TNF-alpha. However, the joint immune response would enter a vicious circle where the inflammation would produce more antigens to be processed resulting in the possible perpetuation of the response.18,19 Anti-CCP antibodies have excellent specificity (90%–100%) with very good sensitivity (41%–88%) for diagnosing RA.20 In addition, anti-CCP antibodies can precede the onset of RA for many years, before the first symptoms of the disease start.21 Although several diseases can be confused with RA in its initial stages, especially in cases where RF is not discriminating, it is useful to test for anti-CCP antibodies in these patients. However there can be anti-CCP positivity in a small proportion of patients with psoriatic arthritis and erosive arthritis.22,23
A limitation of this study was the small amount of patients studied as it was undertaken in only one hospital. A consequence of this would be the lack of significance observed between the presence of anti-CCP positivity and gene dose (Table 2, marginal significance for homozygous SE). In addition, one of the environmental factors that has become relevant in recent years and appears to play an important role in the aetiology of RA is periodontitis caused by P. gingivalis bacterial infection.24 We could consider a limitation the fact that this data was not compiled in all the patients with RA included in our study, it being impossible to assess this variable. Another limitation in our results is that we did not include in the study other genes relating to the disease, which we do not test for in our hospital in daily clinical practice.
In conclusion, Latin American mestizo ethnicity, positive SE, positive RF, and SD of more than 20 packs/year, which characterises heavy smokers, are independent risk factors for developing RA with high titres of positive anti-CCP antibodies (>200U/mL). In patients who are SE carriers, smoking intensity is associated more strongly than SD with an increased risk of anti-CCP positivity, and an interaction was observed between the two factors.
Ethical disclosuresProtection of people and animalsThe authors declare that neither human nor animal testing has been carried out under this research.
Data confidentialityThe authors declare that they have complied with their work centre protocols for the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is held by the corresponding author.
FundingNo financing was received to undertake this study.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: García de Veas Silva JL, González Rodríguez C, Hernández Cruz B. Asociación del epítopo compartido, el tabaquismo y la interacción entre ambos con la presencia de autoanticuerpos (anti-PCC y FR) en pacientes con artritis reumatoide en un hospital de Sevilla, España. Reumatol Clin. 2019;15:289–295.